Science
Related: About this forumSeparation of Uranium(VI) from Thorium(IV) Using Unsymmetrical Acidic Phenanthroline Amide
The paper to which I'll refer briefly is this one: Selective Extraction of Uranium(VI) from Thorium(IV) Using New Unsymmetrical Acidic Phenanthroline Carboxamide Ligands Shihui Wang, Xiaofan Yang, Lei Xu, Yujie Miao, Xiao Yang, and Chengliang Xiao Industrial & Engineering Chemistry Research 2023 62 (38), 15613-15624.
Many people, including the authors of this paper, are fond of the Th232/U233 nuclear fuel cycle, in particular when utilized by molten salt reactors. I have no problem with thorium fueled reactors, although I certainly am no longer inclined, as I once was, to favor as "the best" the molten salt reactors based on the eutectic salt known as "FLiBe" (From the chemical symbols of Fluorine Lithium and Beryllium). This said, the worst nuclear reactor is superior to the best dangerous fossil fuel plant or to systems dependent on fossil fuel plants, for instance unreliable land and mass intensive systems that are inexplicably popular, but otherwise useless.
Overall, for reasons of geochemistry, I generally favor the uranium/plutonium cycle, since I consider it inexhaustible whereas the geochemistry of thorium oxide is somewhat problematic even though, as the paper notes, the terrestrial ores (some of which are lanthanide ores mined to support the useless wind industry, with the radioactive thorium being dumped with the tailings) are more plentiful. Nonetheless, thorium fuels can and should help increase uranium utilization, particularly when used with the existing CANDU type heavy water reactors to achieve very high burnups.
These high burnups in CANDUs will require separation of thorium from uranium for the reprocessing of used nuclear fuels, as well as in molten salt reactors (MSRs). Hence this paper.
From the introduction:
232Th as a fertile material undergoes neutron capture reaction and subsequent beta decays to produce fissile 233U, which is also accompanied by the production of highly radioactive 232U in small amounts. (16,17) For the bred 233U, the portion directly fissions in the reactor, while the nonfissional portion is removed from spent fuel and recycled as new fuel. Therefore, it is necessary to separate uranium from the irradiated thorium to enhance the utilization efficiency of thorium and reduce the volume of nuclear waste. (18) The separation of highly radioactive 232U produced by irradiation will also help to achieve the safe utilization of nuclear energy. Unlike uranium/plutonium separation in the reprocessing of uranium-based spent fuel, the uranium/thorium separation is more challenging because of the invariable oxidation state of thorium. (19) There has been extensive research in the separation of U(VI) and Th(IV), developing various technologies including solvent extraction, (20,21) adsorption, (22,23) ion exchange, (24) and so on, among which the solvent extraction method shows satisfactory performance because of the large processing capacity, high efficiency, and low cost. Until now, the Thorex (thorium–uranium extraction) process developed based on the Purex (plutonium uranium reduction and extraction) process is the only viable process to be applied in the industry, in which U(VI) and Th(IV) are coextracted by 5% tri-n-butyl phosphate (TBP) (Figure 1a) and then purified through ion exchange or precipitation. (25) The study on extractants possessing branched alkyl substituents such as tri-sec-butyl phosphate (TsBP) (26) and tris-2-ethylhexyl phosphate (TEHP) (27) has also been derived based on TBP. However, the organophosphorus extractants can only achieve the coextraction of U(VI) and Th(IV). In addition, the presence of phosphorus in the extractants will inevitably lead to secondary pollution because the extractants cannot be combusted completely...
The authors evaluate a number of extractants:
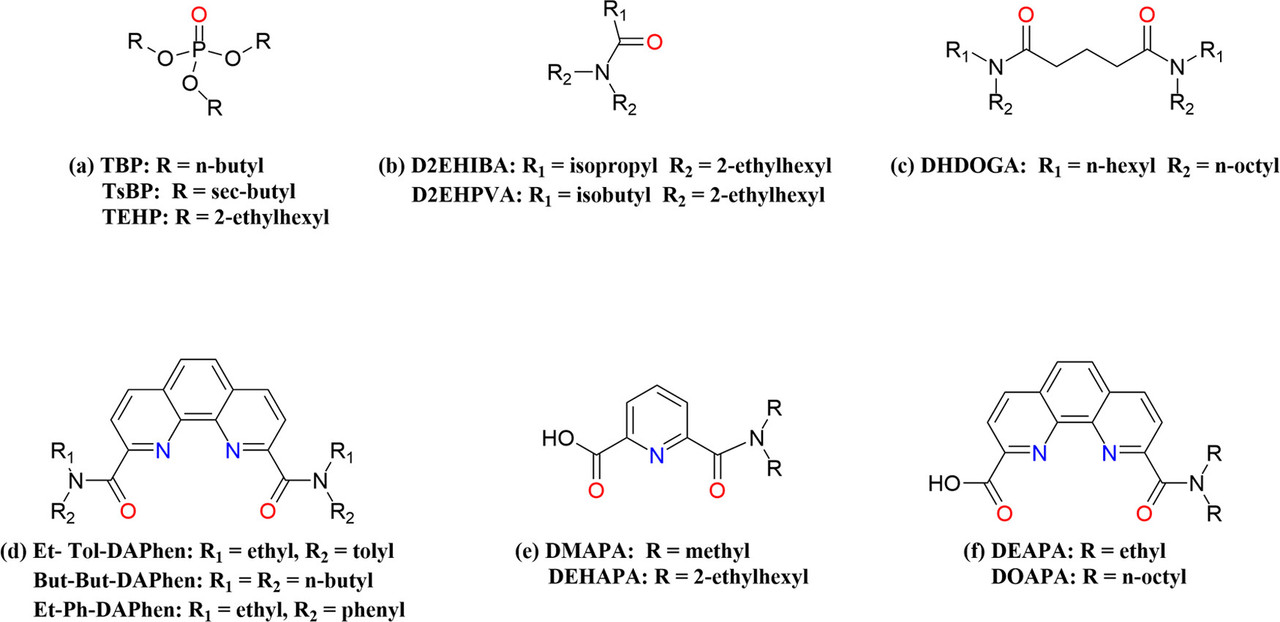
The caption:
The synthetic scheme for the unsymmetric phenanthrolines is shown:
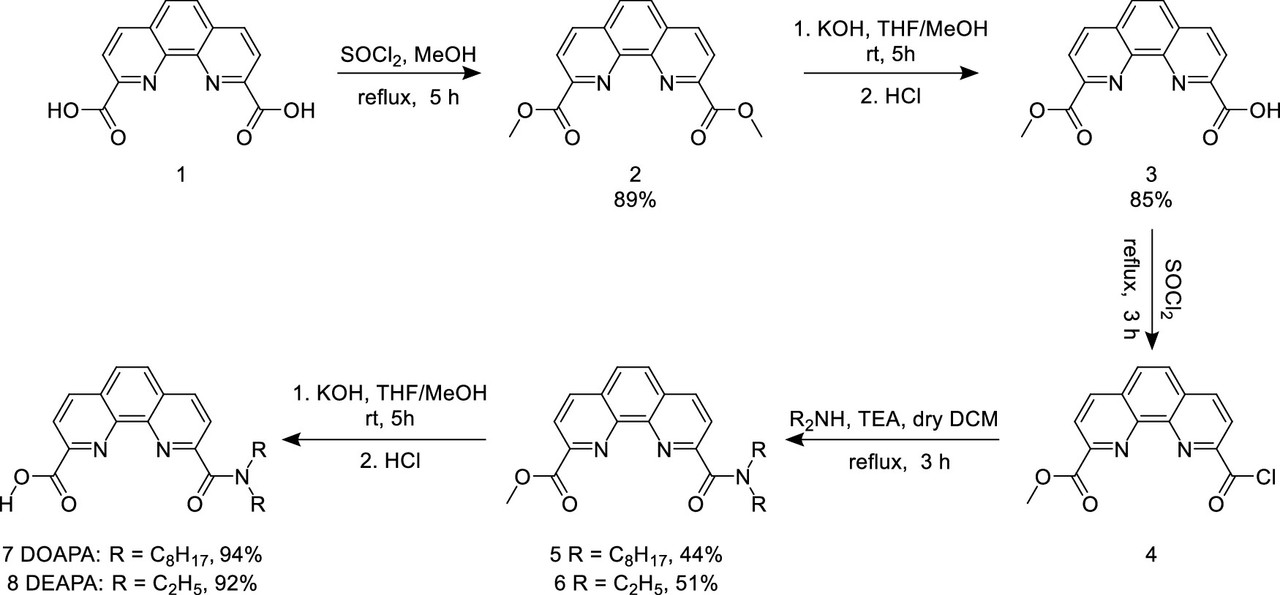
The caption:
The conversion of 2 to 3 is obviously kinetically controlled.
It appears that the desired separations are very good.
From the conclusion to the paper:
Just as I am not really a Th232/U233 kind of guy, although I certainly do not object to thorium fuels which definitely, to my mind, have a place, especially if we wish to do away with terrestrial uranium mining, as seems very possible, and indeed preferable to me, I am not really a solvent extraction kind of guy either. I favor electrochemical/pyroprocessing sort of schemes with anodic dissolution, but the worst nuclear refining scheme is superior to the best fossil fuel refining scheme.
I trust you're having a pleasant weekend.