Environment & Energy
Related: About this forumKinetic Modeling of the Sulfur Iodine Process for Thermochemical Water Splitting to Produce Hydrogen
Last edited Sun Jun 17, 2018, 07:51 PM - Edit history (1)
The paper from the primary scientific literature in this post is this one: Building and Verifying a Model for Mass Transfer and Reaction Kinetics of the Bunsen Reaction in the Iodine–Sulfur Process (Zhang et al Ind. Eng. Chem. Res., 2018, 57 (23), pp 7771–7782).
The "Sulfur Iodine Process" sometimes called the "Sulfur Iodine Cycle" or "SI cycle" or (herein) the "IS process" is a process for splitting water using heat, and thus is vastly thermodynamically more efficient than electrolysis and almost infinitely cleaner, depending on the source of heat, the primary energy, than the process by which 99% of the hydrogen on this planet is produced today, the steam reforming of dangerous natural gas or dangerous coal.
There are many thermochemical water splitting processes by the way, and over the years I've familiarized myself with many of them.
The paper is product of scientists at Institute of Nuclear and New Energy Technology, Tsinghua University, Collaborative Innovation Center of Advanced Nuclear Energy Technology, Beijing, 100084, China. For those who don't know, in international rankings, Tsinghua is known to be one of the greatest universities on this planet, and is sometimes ranked in international rankings higher than MIT, depending on the ranking criteria. The people who do research there are smarter than I am, and, I'm sure in many cases among readers, smarter than you are.
Nevertheless, I still feel free to disagree with the last sentence in their opening paragraph:
I personally believe other thermochemical cycles may be more promising, including some involving boiling metals or nanoceramics in flow cells, but that's just my opinion, and again, I'm not that smart.
In any case a 10MW high temperature gas cooled nuclear reactor has operated at Tsinghua University since the year 2000. It's a "pebble bed" type reactor modeled on German technology developed before Germany went "Energy Stupid." It's not my favorite kind of nuclear reactor, but it works.
The Chinese are smarter than we are because they built the reactor in the first place, and it was a new reactor in this century.
I had heard that Chinese scientists were going to fit this reactor to demonstrate the "SI cycle," but haven't kept up with progress in that area, but apparently the process is still getting significant consideration there, as demonstrated in this very recent paper.
The authors describe the "IS process" thusly:
The IS process consists of the following three chemical reactions: (5)
Bunsen reaction: I2 + SO2 + 2H2O = H2SO4 + 2HI
HI decomposition: 2HI = H2 + I2
Sulfuric acid decomposition: H2SO4 = SO2 + 1/2O2 + H2O
The net reaction of the above-mentioned chemical reactions is water decomposition (H2O = H2 + 1/2O2).
Actually in many accounts, what is called the Bunsen reaction above can be actually divided into two separate reactions with the intermediate being sulfuryl iodide, SO2I2, not to be confused with thionyl iodide, a sulfur species in a lower oxidation state. In theory and perhaps in practice, this intermediate could be isolated. I believe that like its chlorine analog, it's a distillable liquid.
An putative advantage of the SI cycle is that most of the materials in it are either liquids or gases, with the possible exception of iodine, although if you have ever worked with free iodine, you have noted that it appreciably sublimes, a gas phase is always above the solid phase, a situation that is also observed with liquid elemental bromine. (There are many variants of bromine based thermochemical cycles by the way.)
The authors discuss these properties, the phase related systems in their text considering how these phase relations affect kinetics that is, the speed at which the process can operate, which is the focus of their beautiful paper. They write, describing the focus of their work:
A graphic from the paper touches on some issues with phase behavior that they examine:

The caption:
In phase interfaces in chemical processes surface area plays a huge role, and hence the discussion of thin films.
The authors construct models for various aspects involved in the kinetics of this system and produce some graphics involved in considerations of various reaction conditions comparing the model with experimental conditions obtained.
For example:

The caption:
...and...

The caption:
Then there are some Henry's law graphics about mass transfer:

The caption:
concentrations.
More mass transfer:

The caption:
Figure 8. Comparison between the model and the experimental results under different initial pressures and I2 concentrations.
Figure
If you are a scientist you already know this, but if you aren't, and find this topic interesting, this might be helpful.
Anything that is flammable is thermodynamically unstable. Wood, for example, in an oxygen atmosphere would rather be carbon dioxide and water, but is stuck being wood because of kinetics, and kinetics in turn is determined by activation energy required to start it. When you burn wood, you provide this activation by using a match, and the match in turn, is ignited by the energy input from friction when you strike it. Because some thermodynamically favorable reactions require energy inputs to get them started, they are able to persist for long periods of time, we say they are "metastable." You by the way, are metastable. So are diamonds at ordinary temperatures and pressures; diamonds are not forever.
One of the first Nobel Laureates, Svante Arrhenius, found a way to determine the activation energy to make thermodynamically favorable reactions like the combustion of wood happen. It is called the Arrhenius equation, after its discoverer and it remains more than a century after its discovery one of the most important equations there is. It is exponential in format, but is often treated as its natural logarithm which makes it essentially linear:
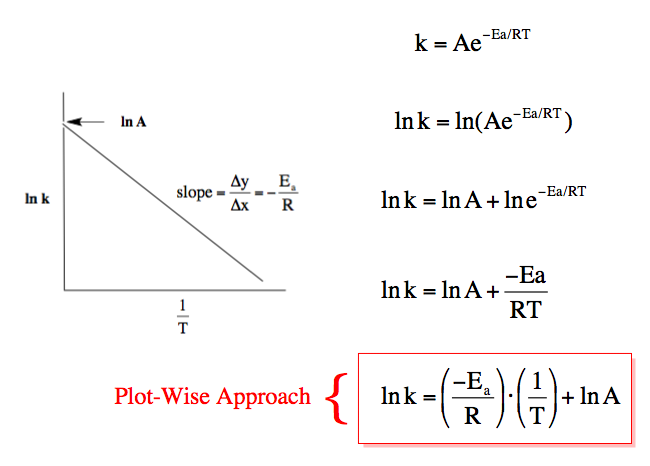
The authors construct Arrhenius plots for the Bunsen reaction:

The caption:
And they find the rate equation for the reaction:

The authors conclude:
Whether you believe it or not - despite whatever horseshit you've heard - the conversion of nuclear energy into chemical energy without the intermediate use of electricity is one of the key technologies for the creation of a sustainable and just world, which is, regrettably, not even close to the world in which we live.
These Chinese scientists irrespective of who pays them or the government under which they live are working in service of humanity, even as the world lives increasingly under a dictatorship of self serving mindless fools.
I am thankful this work is being done.
I am having the best Father's day in my life as a father, since both my sons are doing very well at the things they love, which is all for which a father can hope. If you are a father, I wish you the same.
StevieM
(10,500 posts)I am sure I will get some details wrong here, since my scientific knowledge (and literacy) is nowhere close to your level.
You want to build generation IV nuclear power plants. During the day, when demand is high, we will use them to produce electricity. At night we will use them to produce nuclear heat, which will then be used to create a thermochemical process that splits water, thereby producing hydrogen. The hydrogen will be combined with carbon, from a nearby carbon capture plant, which will produce methanol. The methanol will then be dehydrated in order to produce DME.
Is that right? Or close to right?
I hope you have a good Father's Day weekend, NNadir.
NNadir
(33,515 posts)To understand a little better what I have in mind, perhaps I can discuss positive aspects of two forms of linked energy I strongly oppose, dangerous natural gas and wind energy.
In the last several decades, owing to advances in materials science, specifically the development of thermal barrier coatings originally developed for jet engines, a new type of dangerous natural gas plant has been developed, the "combined cycle" type plant.
This plant uses two different kinds of thermal cycles, one a Brayton cycle - the same cycle used in jet engines in which a gas is compressed and then thermally heated, and allowed to expand in some way - and the second a Rankine cycle, which is the familiar water boiling cycle. The heat to drive the Rankine cycle comes from the exhaust gases of the Brayton cycle. The compressed gas in the Brayton is a mixture of dangerous natural gas and air, when it burns and expands against a turbine it is well over 1000C, and when it is emerges it is slightly cooler but still well over hot enough to boil water.
The combination of the two allows the plant to operate at much higher efficiency than a traditional "Rankine only" power plant. The latter might typically run at 30-35% depending on the temperature of the surroundings, whereas the former might operate at thermal efficiencies as high as 60%.
One of my favorite papers about the wind industry is one written by Wind advocate Paul Denholm years ago, this one: Emissions and Energy Efficiency Assessment of Baseload Wind Energy Systems (Denholm et al, Environ. Sci. Technol., 2005, 39 (6), pp 1903–1911)
I like it because it's considerably more honest than most papers promoting the useless, expensive, and environmentally unacceptable wind industry inasmuch as it plainly admits that from a climate change perspective nuclear energy is cleaner than stored wind energy by a factor of 4, but gives as a rationale for preferring wind that - to put my spin on it - that the public is stupid and doesn't know what's good for it, hates nuclear energy and therefore has to use inferior technology that is worse for the environment.
The storage system he proposes is a CAES system, for "Compressed Air Energy Storage" system.
Now, when you compress air, it gets hot, and if that heat escapes, as surely it will at least in most kinds of systems, you lose energy. Denholm proposes to recover that energy by reheating the air with dangerous natural gas. He indicates a CO2 external cost for this system of 100 g CO2/kwh, about 1/5 that of a pure gas plant, but still unacceptable to my mind.
Now let's turn to the nuclear case. I'm not really an "SI cycle" kind of guy anymore, so I'll propose to use a different cycle, or a modification of it, by referring to a paper written for the solar thermal approach, this one: Thermal ZnO dissociation in a rapid aerosol reactor as part of a solar hydrogen production cycle. (Perkins et al, Int. J. Hydrogen Energy, Volume 33, Issue 2, January 2008, Pages 499-510) The solar part of the paper is garbage and won't work or be economically viable, but the thermochemical cycle itself is very interesting and can easily be adapted to very high temperature nuclear reactors.
In the modification I would make to this cycle, about which I've thought a lot, there would be two separate gas streams created, one being pure hydrogen, the other being an equimolar mixture of oxygen and carbon dioxide, as opposed to what's in the paper, where the carbon dioxide is missing. I also have catalytic ideas in mind that will lower the required temperatures for zinc oxide decomposition to a more reasonable (but still high) temperature.
Now when formed, each of these gas streams would be very hot, easily hot enough to expand against a turbine, either to operate a compressor or generate electricity as a side product, thus giving a Brayton type cycle. Moreover they might well still be hot enough when exiting the turbine to boil water, giving an opportunity for a Rankine cycle.
The high thermodynamic efficiency will be a product of the very high temperature at which the gases are created; thermodynamic efficiency being a function of the temperature difference between the heat of the working fluid and the surroundings, which is why power plants are more efficient in winter.
However we're not done. The oxygen carbon dioxide mixture has a high energy content if it is heated over a carbon source, say biomass, in which the carbon source is derived from air. If this system is closed and smoke stack free, I have convinced myself that it can be converted to a mixture of carbon oxides and hydrogen, syn gas, the starting product for DME synthesis. (This approach might also be used to generate pure hydrogen, depending on the industrial needs at the time.)
People don't realize this generally, but syn gas is actually higher energy than its products, in my preferred case, DME. This mixture is metastable, but higher energy. Thus when the mixture is passed over a catalyst, considerable heat is generated, and the products are DME and water. If this exhaust, DME and water, expands against a turbine we can generate electricity, not necessarily continuously but at periods of peak demand. As the water condenses, rejecting heat to the surroundings, the pressure gradient to drive the turbine is maintained, and if the turbine is also mechanically attached to a compressor, the DME can be liquified and stored for later use.
In this case, because of the second law of thermodynamics, some of the original nuclear energy will be lost to the surroundings, however much of it can be recovered as work.
I note that there are still further recoveries possible, since liquified DME is a refrigerant, but perhaps this is more than you wanted to know.
Thanks for your Father's day wishes. I am having a wonderful Father's day because I'm very proud and inspired by the work my two sons are doing, one overseas in France doing materials science research, and the other right here in New Jersey, doing art.
Thanks as well for your question.
Have a wonderful day yourself.
NNadir
(33,515 posts)The Bunsen reaction was copied directly from the paper, but apparently there's a typo, leading to incorrect mass balance for the equation and the sum of the equations, the dissociation of water.
It reads (and read in the original):
It should read:
I'll take the liberty of correcting it in the OP.