Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Science
Related: About this forumInvasive mosquitoes essentially eliminated from two Chinese Islands by combined biobased attack.
The paper I'll discuss in this post is this one: Incompatible and sterile insect techniques combined eliminate mosquitoes (Xi, Zhang, & Zheng, Nature Online Published July 17, 2019)
A Nature news item, which I believe should be open sourced discussing the paper is here: World’s most invasive mosquito nearly eradicated from two islands in China (Nature, July 17, 2019)
From the abstract and opening paragraphs of the full paper:
The radiation-based sterile insect technique (SIT) has successfully suppressed field populations of several insect pest species, but its effect on mosquito vector control has been limited. The related incompatible insect technique (IIT)—which uses sterilization caused by the maternally inherited endosymbiotic bacteria Wolbachia—is a promising alternative, but can be undermined by accidental release of females infected with the same Wolbachia strain as the released males. Here we show that combining incompatible and sterile insect techniques (IIT–SIT) enables near elimination of field populations of the world’s most invasive mosquito species, Aedes albopictus. Millions of factory-reared adult males with an artificial triple-Wolbachia infection were released, with prior pupal irradiation of the released mosquitoes to prevent unintentionally released triply infected females from successfully reproducing in the field...
From the paper's body:
SIT, in which artificially reared radiation-sterilized males are released into the field to mate with wild females—thereby preventing them from producing viable offspring—has successfully suppressed populations of several insect pests of agricultural and veterinary importance1. However, despite various trials, SIT has not been widely used against mosquitoes because of the difficulty of irradiating males without reducing their mating competitiveness and survival2,3,4. A promising alternative approach is the related IIT5, in which released males are infected with the maternally inherited endosymbiotic bacteria Wolbachia, resulting in sterile matings with field females that are not infected with the same Wolbachia strain, a phenomenon known as cytoplasmic incompatibility6,7. An advantage of IIT is that Wolbachia-based sterilization has little or no effect on male mating competitiveness and survival8,9,10. Historically, in a small-scale pilot field trial IIT successfully eradicated the primary filariasis vector Culex quinquefasciatus5—although another trial showed limited success11—but the approach has not been deployed operationally, primarily because the accidental release of fertile females risks causing population replacement, whereby individuals infected with the same Wolbachia strain as released males replace the wild-type field population, preventing future population suppression (as matings between released males and field females are no longer incompatible)11,12,13......
The mosquito in question is the one that transmits Zika virus and Dengue fever, both of which can be expected to increase their range because of our disinterest in addressing climate change:
The globally invasive mosquito A. albopictus is an important vector of arboviruses—including dengue and Zika viruses—that is particularly challenging to control using traditional approaches27,28. Unlike some other mosquito vectors, A. albopictus is superinfected with two native Wolbachia strains (wAlbA and wAlbB), complicating the development of Wolbachia-based control strategies. Various Wolbachia strains have previously been artificially introduced into A. albopictus29,30,31,32, including wPip in mosquitoes cured of their native double wAlbA/wAlbB infection33, but these endosymbiont–host associations are either unsuitable for IIT—as they are pathogenic or do not inhibit arboviruses—or their appropriateness has not been fully determined. Here we report the generation and characterization of an A. albopictus line (termed HC) with an artificial triple-Wolbachia infection, and demonstrate its use in an open-release field trial of the combined IIT–SIT approach.
The authors developed a transgenic mosquito that was susceptible to three strains of Wolbachia bacteria by inserting genes from another mosquito into the target species:
For use in IIT, Wolbachia must induce high levels of cytoplasmic incompatibility to effectively sterilize wild females, and have high maternal transmission to enable efficient mass production of only infected males for release as well as low fitness costs to ensure that released males can mate competitively with respect to wild males. In addition, as a responsible safety precaution, any released mosquitoes should have a lower vector competence for human pathogens than the target field population34. Accordingly, we created an endosymbiont infection appropriate for IIT by transferring wPip from its native mosquito host Culex pipiens into the A. albopictus HOU line by embryonic microinjection21, generating the mosquito line HC, which possesses a triple-Wolbachia infection (the artificially transinfected wPip as well as the original native wAlbA and wAlbB strains)
There is a risk of the transgenic females escaping into the environment and the gene spreading in such a way as to reduce the effectiveness of the technique, therefore a rigorous program of separating females - largely manual - was employed and the females were all irradiated to render them sterile. For this species of mosquito irradiation of males does not produce the desired effect because the irradiated males are not as competitively successful in mating with the females as the unirradiated males, thus are not able to have a huge effect on reducing the mosquito populations.
This approach apparently worked better:
Some graphics:
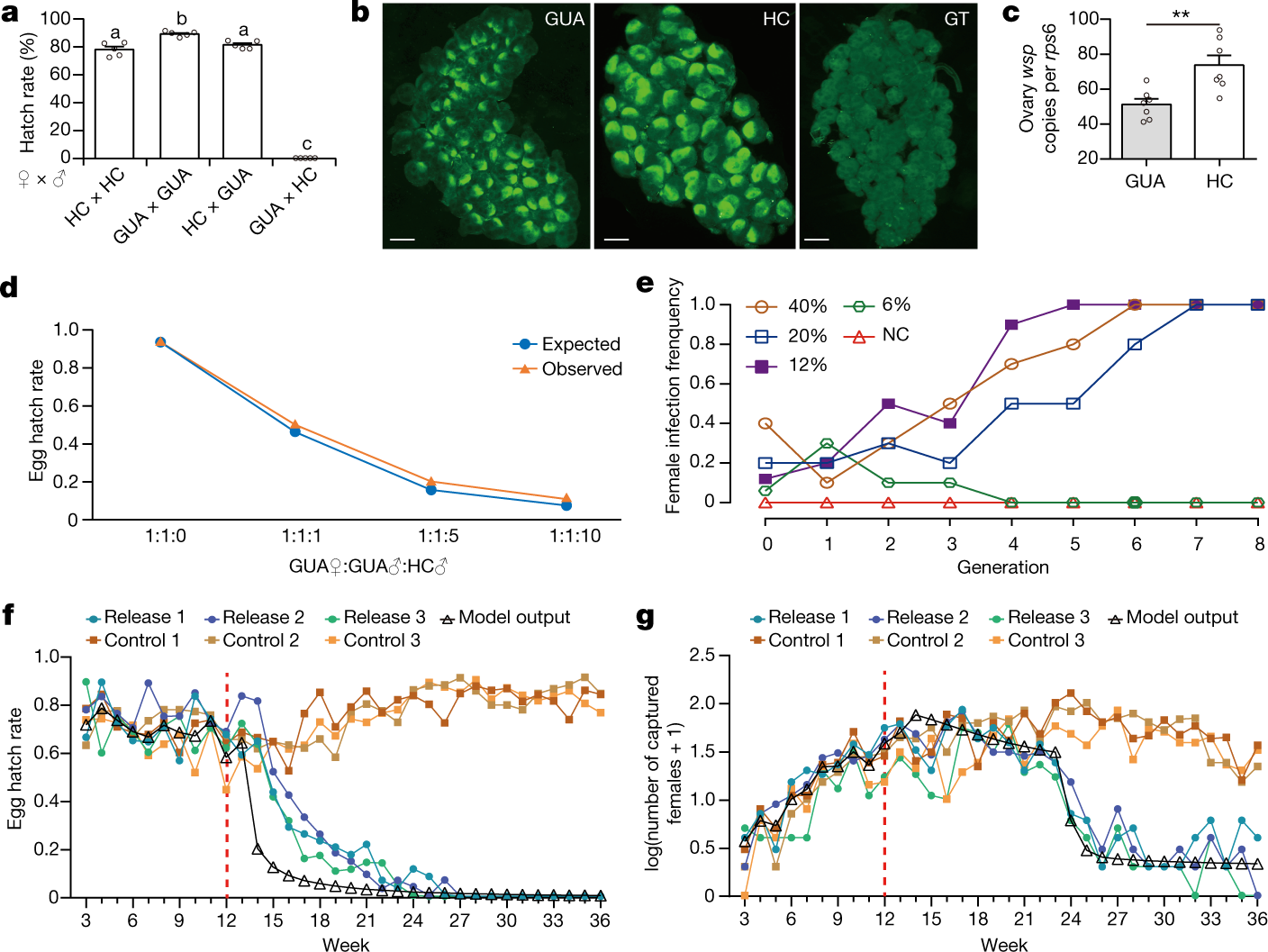
The caption:
a, Reciprocal crosses between HC and GUA lines. Letters above columns indicate significant differences between groups (mean ± s.e.m.; n = 5 for each cross, ANOVA and Tukey’s multiple comparisons test, F(3, 16) = 513.5, P < 0.0001). b, Fluorescence in situ hybridization (FISH), showing Wolbachia distribution and density in ovaries. Scale bar, 100 µm. c, Real-time quantitative PCR (RT-qPCR) analysis of the relative number of Wolbachia wsp gene copies (mean ± s.e.m.; n = 7 pools of two ovary pairs for each group, two-sided Mann–Whitney test, P = 0.006). d, Egg hatch rate in laboratory cage populations with different GUA female:GUA male:HC male ratios. Two-sided binomial test: n = 3,681, P = 0.0002 (1:1:1); n = 4,083, P < 0.0001 (1:1:5); n = 2,392, P = 0.0009 (1:1:10). e, Invasion of wPip in laboratory GUA populations after a single release of different numbers of HC females at generation 0. For release of 6% and 12% HC females, a single simultaneous inundative release of HC males at a 4:1 ratio with GUA males was also used, to mimic accidental female release during IIT. NC, negative control. f, g, Combined IIT–SIT in semi-field cages: egg hatch rate (f) and adult female population sizes (g). Target GUA populations were established in six replicate cages for 12 weeks before the release (indicated by the dashed red lines) of HC males, with HC females to mimic female contamination. Black triangles represent mathematical model outputs (mean goodness of fit: egg hatch rate R2 ? 0.9325; number of females captured R2 ? 0.8417).
 ?as=webp
?as=webp
The caption:
a, b, Satellite images of control and release sites 1 (a) and 2 (b) in Guangzhou city (map data: Google, DigitalGlobe). c, Release schedule. d, e, Effect of HC male release on A. albopictus larval stages in release sites 1 (d) and 2 (e). Vertical green dashed lines indicate onset of HC release. Red dashed line in d indicates that only IIT was used in release site 1 in 2015. Red solid lines in d and e indicate period of combined IIT–SIT in both release sites in 2016 and 2017. Two-sided Mann–Whitney test. Pre-release period: site 1 2014, n = 22, P = 0.164; site 2 2015, n = 26, P = 0.0805. Release period: IIT only: site 1 2015, n = 34, P < 0.0001; 12 March–21 May, n = 11, P = 0.0032. Release period: combined IIT–SIT: site 1 (2016, n = 37, P < 0.0001; 2017, n = 34, P < 0.0001); site 2 (2016, n = 32, P < 0.0001; 2017, n = 35, P < 0.0001).

The caption:
a, b, Relative density of adult females collected weekly in control and release sites 1 (a) and 2 (b). The red dashed line in a indicates period of IIT only in 2015. Red solid lines in a and b indicate the period of combined IIT–SIT in 2016 and 2017. Two-sided Mann–Whitney test. Site 1 2015, n = 34, P < 0.0001; site 1 2016, n = 37, P < 0.0001; site 1 2017, n = 37, P < 0.0001; site 2 2016, n = 37, P < 0.0001; site 2 2017, n = 38, P < 0.0001. c, d, Spatial dynamics of adult suppression at release sites 1 (c) and 2 (d) during the dengue transmission season in Guangzhou in 2017.
 ?as=webp
?as=webp
The caption:
a, Number of HC males released weekly and observed ratios of HC to wild-type males in the field at release site 1. Blue dashed line indicates target overflooding ratio of 5:1. b, Comparison of observed and expected weekly egg hatch rates at release site 1. Two-sided paired t-test after arcsine transformation. 2015, n = 27, P = 0.6522; 2016, n = 31, P < 0.0001; 2017, n = 33, P < 0.0001. c, Total number of HC males produced weekly and female contamination rate at adult stage in mass rearing facility. d, Comparison of monthly positive female rate detected in HC males in mass rearing facility (laboratory quality control) and that observed in adults collected from the field. Two-sided paired t-test after arcsine transformation: Laboratory (n = 19) versus site 1 (n = 18), P < 0.0001; versus site 2 (n = 16), P = 0.0012. Pearson correlation: site 1, r = 0.110, n = 18, P = 0.664; site 2, r = 0.839, n = 16, P < 0.0001.
 ?as=webp
?as=webp
The effect of release on public support for the project:
The caption:
a, Pie chart showing community support for the field trial in release site 1 before (13.0%, n = 123 interviews) and after (54.3%, n = 431) mosquito releases (?2 = 71.29, P < 0.0001). b, Mosquito human landing catches in release and control sites 1 and 2, July to November 2017. Mean ± s.e.m.; n = 4 independent biological replicates for both control and release sites; two-sided paired t-test. Site 1, t = 6.988, 3 degrees of freedom, P = 0.006; site 2, t = 3.566, 3 degrees of freedom, P = 0.0376
.
Conclusions from the paper:
In conclusion, combined IIT–SIT nearly eliminated two field populations of A. albopictus over a two-year period. The few mosquitoes remaining were probably migrants from outside the study area, as indicated by population genetic analyses43 and their presence in areas with good transport links, whereas isolated areas were mosquito-free. The possibility of population replacement emphasizes the importance of releasing mosquitoes that cannot increase pathogen transmission. As shown here, wPip markedly reduces arbovirus transmission by wild-type A. albopictus with their native double wAlbA and wAlbB infection, so unintended population replacement could even be beneficial in the short- and long-term, by initially collapsing vector populations and rendering any newly established populations incompetent for pathogen transmission. However, the aim of population suppression is preferable and has greater public acceptance, as it enables reduction of nuisance biting and pathogen transmission, and long-term mosquito eradication in the absence of immigration. The combined IIT–SIT approach is environmentally friendly and cost-effective (see Supplementary Information), enabling vector control in complex and inaccessible urban habitats in which implementation of standard vector control is difficult27,28, as released males actively seek wild females, and allows release of much higher numbers of male mosquitoes in comparison to IIT alone, while simultaneously protecting against accidental female release. Area-wide application of this approach will necessitate the development of novel technologies, especially with regard to scaling-up production capacity and enabling efficient mass release of mosquitoes.
It is important to note that this mosquito is an introduced species in many areas, and it is a transmitter of the Zika virus which will produced severely disabled children.
Other techniques are being explored to address parasite vectors, including those which transmit Malaria. These technologies also involve genetic engineering and are somewhat more controversial, in particular the technology involving "gene drives."
Gene drives are discussed in a news item in the previous issue of Nature: Self-destructing mosquitoes and sterilized rodents: the promise of gene drives
Altering the genomes of entire animal populations could help to defeat disease and control pests, but researchers worry about the consequences of unleashing this new technology.
I am not particularly competent in this particular technology so as to be able to address it with strong opinions, but as invasive species are a huge environmental problem, and as human disease compromises human development goals which, in my view, extend to environmental degradation and war and other noxious human conditions, I am keeping an open mind.
InfoView thread info, including edit history
TrashPut this thread in your Trash Can (My DU » Trash Can)
BookmarkAdd this thread to your Bookmarks (My DU » Bookmarks)
1 replies, 659 views
ShareGet links to this post and/or share on social media
AlertAlert this post for a rule violation
PowersThere are no powers you can use on this post
EditCannot edit other people's posts
ReplyReply to this post
EditCannot edit other people's posts
Rec (4)
ReplyReply to this post
1 replies
 = new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
= new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
Invasive mosquitoes essentially eliminated from two Chinese Islands by combined biobased attack. (Original Post)
NNadir
Jul 2019
OP
NCjack
(10,279 posts)1. I nominate the barrier islands and bays of North Carolina for intensive testing -- Please. nt