Seeking Life's Origins, a Non-Enzymatic Route to Peptide Synthesis In Water Is Discovered.
The paper I'll discuss in this post is this one: Peptide ligation by chemoselective aminonitrile coupling in water (Powner, Islam, and Canavelli, Nature 571, 546–549 (2019))
Great fundamental scientific questions remain mysterious, and among the greatest is the question of how life came to exist, and by extension, how we came to exist. The great strides in molecular biology that began accelerating rapidly in the 1980's up to the present day have come so far that we are now at the cusp of being able to design life, even synthesize life, but the question still remains about how life arose spontaneously, without a designer, given the great complexity that life now exhibits as a set of closely interacting highly complex molecules.
(Please note that this statement does not imply that the absence of an extant explanation does not imply intelligent design anymore than a lack of an explanation for the heat of the sun, which was a complete mystery until the relatively recent discovery of nuclear reactions, implied the necessity for the existence of Apollo. A question lacking an answer does not imply an arbitrary answer must be true.)
Many interesting theories have been advanced, going back to the Miller experiment in the 1950's, where amino acids, the constituents of proteins, were synthesized from a putative mixture that was believed to represent the Earth's early atmosphere subjected to electrical discharges (lab lightening) and high energy radiation. However these amino acids lacked the asymmetry that characterized all living things we observe today (with some rare exceptions to the case) and the origin of asymmetry itself remains as much a question as the origin of the sun's heat presented as late as the 1930's.
It is now known that asymmetric molecules exist in meteorites suggesting they are available outside of Earth bound processes; it may be that this asymmetry may be tied to certain asymmetries observed in nuclear reactions. It is also known that purines and pyrimidines, which represent the cores of nucleic acids are also found in space, and there is some evidence that primitive sugars - sugars also essential to nucleic acids - also exist in space. The Nobel Prize generating discovery that RNA could do what proteins do, catalyze chemical reactions (awarded to Thomas Cech and Sydney Altma) suggested evidence - but not proof - "chicken and egg" argument, since the formation of proteins is dictated by nucleic acids in living systems now observed, and the synthesis of nucleic acids like RNA and DNA catalyzed by proteins.
One of the greatest mysteries is how amino acids can couple, form peptide bonds in water. Human beings can now couple amino acids on an industrial scale - I've participated in such projects - and peptides, sequences of amino acids that make up proteins having scores of such bonds have been synthesized on a ton scale. The chemistry however under which this is done avoids water, in fact can be said to remove water, and is often conducted using solvents and reagents that would be quite hostile to living systems, and in any case, is unlikely in a prebiotic world.
This brings us to the paper evoked at the outset of this post; by looking at some artifacts of how life still operates, using sulfur based oxidation, the authors have synthesized peptide bonds in water.
From the abstract:
Amide bond formation is one of the most important reactions in both chemistry and biology1,2,3,4, but there is currently no chemical method of achieving ?-peptide ligation in water that tolerates all of the 20 proteinogenic amino acids at the peptide ligation site. The universal genetic code establishes that the biological role of peptides predates life’s last universal common ancestor and that peptides played an essential part in the origins of life5,6,7,8,9. The essential role of sulfur in the citric acid cycle, non-ribosomal peptide synthesis and polyketide biosynthesis point towards thioester-dependent peptide ligations preceding RNA-dependent protein synthesis during the evolution of life5,9,10,11,12,13. However, a robust mechanism for aminoacyl thioester formation has not been demonstrated13. Here we report a chemoselective, high-yielding ?-aminonitrile ligation that exploits only prebiotically plausible molecules—hydrogen sulfide, thioacetate12,14 and ferricyanide12,14,15,16,17 or cyanoacetylene8,14—to yield ?-peptides in water. The ligation is extremely selective for ?-aminonitrile coupling and tolerates all of the 20 proteinogenic amino acid residues. Two essential features enable peptide ligation in water: the reactivity and pKaH of ?-aminonitriles makes them compatible with ligation at neutral pH and N-acylation stabilizes the peptide product and activates the peptide precursor to (biomimetic) N-to-C peptide ligation. Our model unites prebiotic aminonitrile synthesis and biological ?-peptides, suggesting that short N-acyl peptide nitriles were plausible substrates during early evolution.
From the introductory body of the paper:
To improve the efficiency and selectivity of peptide ligation in water we sought to develop a mechanism for non-enzymatic peptide synthesis, which would operate via biomimetic N ? C ligation in water at near-neutral pH, and we suspected that a combination of sulfur and nitrile chemistry would be required8,9,14,18,19,20,21 (Fig. 1a). Proteinogenic ?-aminonitriles (AA-CN) are readily synthesized8,18, and their direct ligation would provide the simplest prebiotic pathway to peptides. Unfortunately, incubation of AA-CN in water results in extremely ineffective peptide synthesis22. ?-Amino acids (AA) are widely assumed to be prebiotic precursors of peptides, but the harsh conditions (typically strongly acidic or alkaline solutions) required for AA formation from AA-CN are incompatible with the integrity of both peptides and electrophilic activating agents. Therefore, we sought a more congruent and direct pathway from AA-CN to ?-peptides...
In organic synthesis, a route sometimes used to synthesize carboxylic acids (which amino acids are) proceeds through cyanide, and in fact, an early synthetic route to amino acids, known as the Strecker synthesis is a reaction involving cyanide:

Asymmetric Strecker syntheses of amino acids is well known - I've run many of these myself - but these rely on asymmetric catalysts the ultimate source of which is living things.
The paper continues:
[div class="excerpt"...]Maurel and Orgel have previously suggested that AA-SH16 might offer an interesting alternative to biological thioesters10,11. AA-SH combine excellent aqueous stability with highly selective (electrophilic or oxidative) activation12,14,16,24, but their prebiotic synthesis presents difficulties25 and they undergo inefficient ligation at near-neutral pH (Supplementary Discussion)16,26. To overcome these problems we reconsidered the synthesis of thioacids from nitriles (Fig. 1b). Recently we reported that high-yielding nucleophilic displacement of sulfides by Gly-CN19 is promoted by the low pKaH of AA-CN in water, and we hypothesized that coupling AA-CN to the C terminus of a growing peptide would be facile at neutral pH...
The pictures:
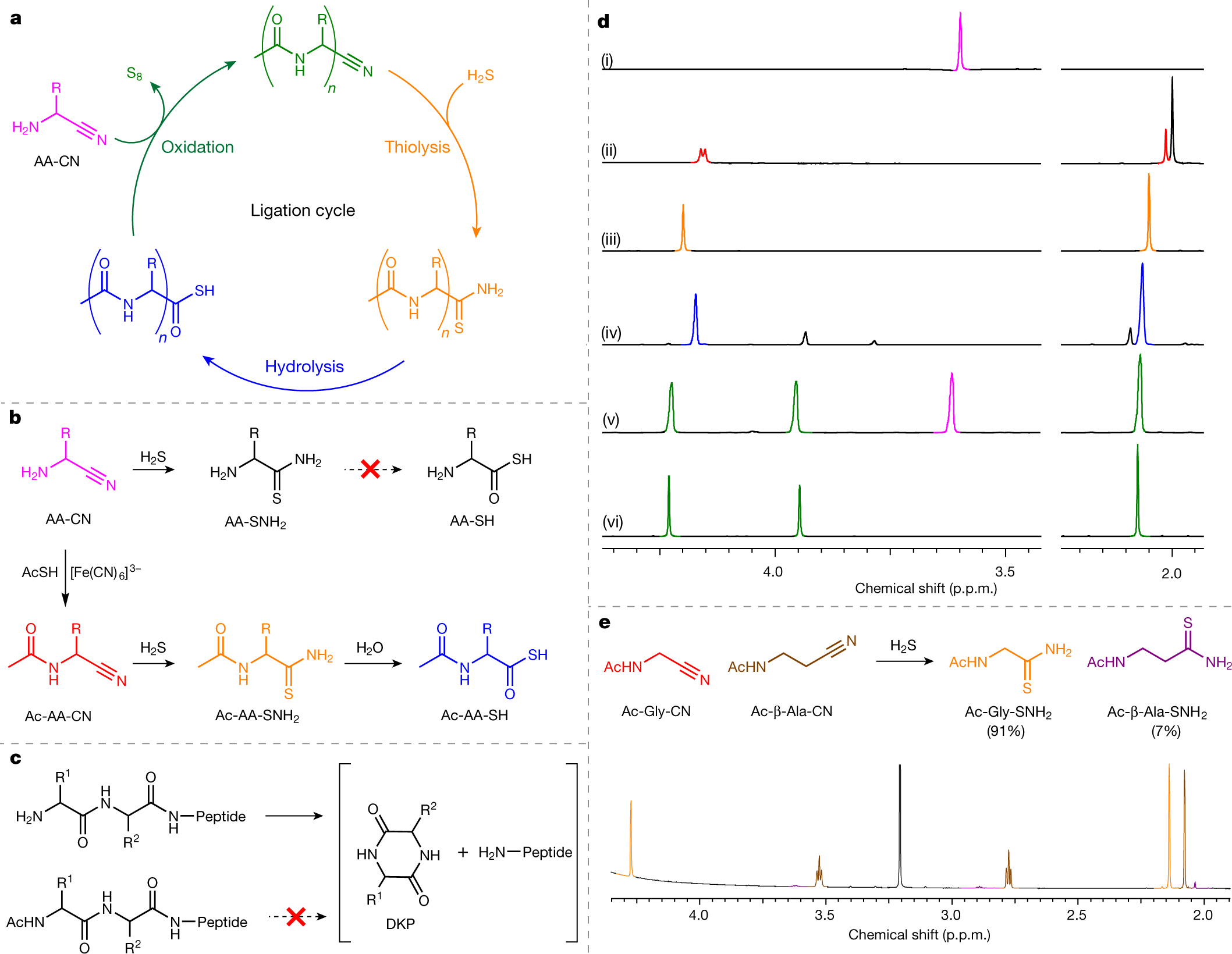
The caption:
a, Iterative AA-CN ligation to give N-acetyl peptide nitriles (Ac-AAn-CN; green) by sequential thiolysis, hydrolysis and AA-CN ligation. b, The thiolysis of AA-CN (magenta) to yield AA-SH (black) is not observed, whereas the thiolysis of N-acetyl aminonitrile (Ac-AA-CN; red) to ?-amidoacyl thioacid (Ac-AA-SH; blue) is facile. c, Iterative truncation of peptides by DKP excision is blocked by N-acylation. d, 1H nuclear magnetic resonance (NMR) spectra (600 MHz, H2O : D2O = 98:2, 25?°C), showing: (i) Gly-CN (magenta); (ii) Ac-Gly-CN (quantitative yield, quant.; red) synthesized by the reaction of Gly-CN (50 mM) with thioacetic acid (3 equiv.) and K3[Fe(CN)6] (9 equiv.) in water at pH 9 at room temperature after 10 min; (iii) Ac-Gly-SNH2 (quant.; orange) synthesized by the reaction of Ac-Gly-CN (50 mM) with H2S (10 equiv.) in water at pH 9 at room temperature after 1 d; (iv) Ac-Gly-SH (81%; blue) synthesized by hydrolysis of Ac-Gly-SNH2 (50 mM) at pH 9 and 60?°C after 1 d; (v) Ac-Gly2-CN (quant.; green) synthesized by the reaction of Ac-Gly-SH (50 mM) with Gly-CN (2 equiv.; magenta) and K3[Fe(CN)6] (3 equiv.) in water at pH 9 and room temperature after 20 min; (vi) pure Ac-Gly2-CN. e, 1H NMR spectrum (600 MHz, H2O : D2O = 98:2, 25?°C) showing the reaction of homologous amidonitriles Ac-Gly-CN (red) and Ac-?-Ala-CN (brown) with H2S (10 equiv., pH 9, room temperature, 1 d), which strongly favours thiolysis of the proteinogenic glycyl residue to yield Ac-Gly-SNH2 (orange) (Supplementary Fig. 19).
There is a fair amount of text in the paper detailing the process, including a well known complex of cyanide, the iron complex ferricyanide (a constituent of the pigment Prussian Blue), but overall the paper suggests a route to prebiotic uncatalyzed peptide synthesis, and as peptides make up proteins - the distinction between a large peptide and a small protein is not entirely well defined - this adds some interesting clues.
From the paper's conclusion:
In a clear departure from the convention that AA are essential for prebiotic peptide synthesis, we have found that their precursors, AA-CN, are predisposed to undergo selective ligation at biochemically relevant pH and concentration. N-Acylation initiates our peptide synthesis strategy and activates a ligated aminonitrile to thiolysis and hydrolysis to its respective ?-amidothioacid. N-Acylation circumvents the irreversible derivatization of peptides by electrophiles (such as COS17; see Supplementary Discussion) and promotes (biomimetic) N ? C peptide ligation. Our peptide ligation strategy requires separate sequential delivery of H2S and an activating agent. For example, H2S and ferricyanide are mutually reactive feedstock molecules and would need to be delivered from separate source locations. However, repeated sequential delivery of H2S and then AA-CN and an oxidant (for example, ferricyanide), chalcophilic metal ion (for example, Cu2+) or an electrophile (for example, cyanoacetylene) would yield controlled stepwise peptide ligation. Controlled synthesis, which responds to environmental or internal stimuli, is an essential element of metabolic regulation, and we speculate that coupling iterative aminonitrile ligation to metabolic (redox) cycles may lead to positive cooperative feedback during the early evolution of life.
Interesting work, I think, perhaps somewhat esoteric, but in these trivializing times, uplifting to see.
Have a nice day tomorrow.


