Crosslinking ionic oligomers as conformable precursors to calcium carbonate
The paper I'll discuss in this post is this one: Crosslinking ionic oligomers as conformable precursors to calcium carbonate (Tang et al, Nature 574, 394–398 (2019))
The fastest growing contributor on this planet in the 21st century has been dangerous coal, followed by petroleum, which is likely to be exceeded in the next decade by dangerous natural gas. The next largest contributor, also accelerating, is land use changes. Following these, closely is concrete. (Much of the climate change cost of concrete is connected to heat, almost always generated by the use of dangerous fossil fuels. In theory, if not in wide practice it is possible for the use of concrete to by carbon negative, and some major advances along this line have been made, for instance Riman Concrete, but even Riman concrete requires heat to make. Nuclear heat is actually the only practical way to make concrete without dangerous fossil fuels, despite whatever cartoons you've read or even written about solar thermal plants. Solar thermal plants didn't work, they aren't working and they won't work to address climate change and they will never work to make concrete.
In the past several years, my vicarious interest in my son's education has led me to consider a concept called "polymer derived ceramics" in which is just what it sounds like, a polymer is, via process engineering (and generally heat) converted into a highly structured ceramic. This paper touches on that concept, at least in a loose way. Beautiful things, these, with all sorts of fabulous potential applications.
The abstract, which is open sourced:
Inorganic materials have essential roles in society, including in building construction, optical devices, mechanical engineering and as biomaterials1,2,3,4. However, the manufacture of inorganic materials is limited by classical crystallization5, which often produces powders rather than monoliths with continuous structures. Several precursors that enable non-classical crystallization—such as pre-nucleation clusters6,7,8, dense liquid droplets9,10, polymer-induced liquid precursor phases11,12,13 and nanoparticles14—have been proposed to improve the construction of inorganic materials, but the large-scale application of these precursors in monolith preparations is limited by availability and by practical considerations. Inspired by the processability of polymeric materials that can be manufactured by crosslinking monomers or oligomers15, here we demonstrate the construction of continuously structured inorganic materials by crosslinking ionic oligomers. Using calcium carbonate as a model, we obtain a large quantity of its oligomers (CaCO3)n with controllable molecular weights, in which triethylamine acts as a capping agent to stabilize the oligomers. The removal of triethylamine initiates crosslinking of the (CaCO3)n oligomers, and thus the rapid construction of pure monolithic calcium carbonate and even single crystals with a continuous internal structure. The fluid-like behaviour of the oligomer precursor enables it to be readily processed or moulded into shapes, even for materials with structural complexity and variable morphologies. The material construction strategy that we introduce here arises from a fusion of classic inorganic and polymer chemistry, and uses the same cross-linking process for the manufacture the materials.
An excerpt from the introduction:
Letter
Published: 16 October 2019
Crosslinking ionic oligomers as conformable precursors to calcium carbonate
Zhaoming Liu, Changyu Shao, Biao Jin, Zhisen Zhang, Yueqi Zhao, Xurong Xu & Ruikang Tang
Nature volume 574, pages394–398 (2019) | Download Citation
Article metrics
556 Accesses
13 Altmetric
Metricsdetails
Abstract
Inorganic materials have essential roles in society, including in building construction, optical devices, mechanical engineering and as biomaterials1,2,3,4. However, the manufacture of inorganic materials is limited by classical crystallization5, which often produces powders rather than monoliths with continuous structures. Several precursors that enable non-classical crystallization—such as pre-nucleation clusters6,7,8, dense liquid droplets9,10, polymer-induced liquid precursor phases11,12,13 and nanoparticles14—have been proposed to improve the construction of inorganic materials, but the large-scale application of these precursors in monolith preparations is limited by availability and by practical considerations. Inspired by the processability of polymeric materials that can be manufactured by crosslinking monomers or oligomers15, here we demonstrate the construction of continuously structured inorganic materials by crosslinking ionic oligomers. Using calcium carbonate as a model, we obtain a large quantity of its oligomers (CaCO3)n with controllable molecular weights, in which triethylamine acts as a capping agent to stabilize the oligomers. The removal of triethylamine initiates crosslinking of the (CaCO3)n oligomers, and thus the rapid construction of pure monolithic calcium carbonate and even single crystals with a continuous internal structure. The fluid-like behaviour of the oligomer precursor enables it to be readily processed or moulded into shapes, even for materials with structural complexity and variable morphologies. The material construction strategy that we introduce here arises from a fusion of classic inorganic and polymer chemistry, and uses the same cross-linking process for the manufacture the materials.
Main
Many materials are consolidated from their crystallized powders16, but their resulting discontinuous internal structures render them brittle with a poor ability to resist fracture17,18. By contrast, polymeric materials are ubiquitous in modern society, due not only to their varied properties but also to their ease of fabrication15,19. The polymerization strategy is superior to crystallization because of its efficiency and controllability. In polymer chemistry, covalent bonds have an important role in ensuring the linkage of small units. Although a few covalent-bond-based inorganic materials (for example silicone and silica)20,21 can be obtained as polymers, there is no general method for the preparation of such materials by crosslinking owing to the lack of investigation into ionic monomers or oligomers for this purpose. In the control of polymerization reactions, a capping agent is key22: capping can stabilize precursors, whereas de-capping can initiate polymerization. Analogously, we proposed that ionic oligomers could be stabilized by an appropriate capping agent. Capping based on hydrogen bonding was thought to be suitable, because most inorganic complexes contain oxygen. For example, triethylamine (TEA) can form a hydrogen bond with a protonated carbonate through its tertiary amine group. More importantly, TEA is a small molecule that can be volatilized at room temperature, and it was expected that this could initiate an expected crosslinking reaction.
The authors use triethylamine in a solvent, the solvent in this case being ethanol (which of course, unless the ethanol is recovered, makes industrialization questionable. The calcium carbonate is made by bubbling carbon dioxide through an ethanolic solution of calcium chloride. Mass spectrometry demonstrated the existence of calcium carbonate polymers from trimers to undecamers, with, for some reason, nonamers excluded. The structures were also studied by 13C NMR.
A figure from the paper:
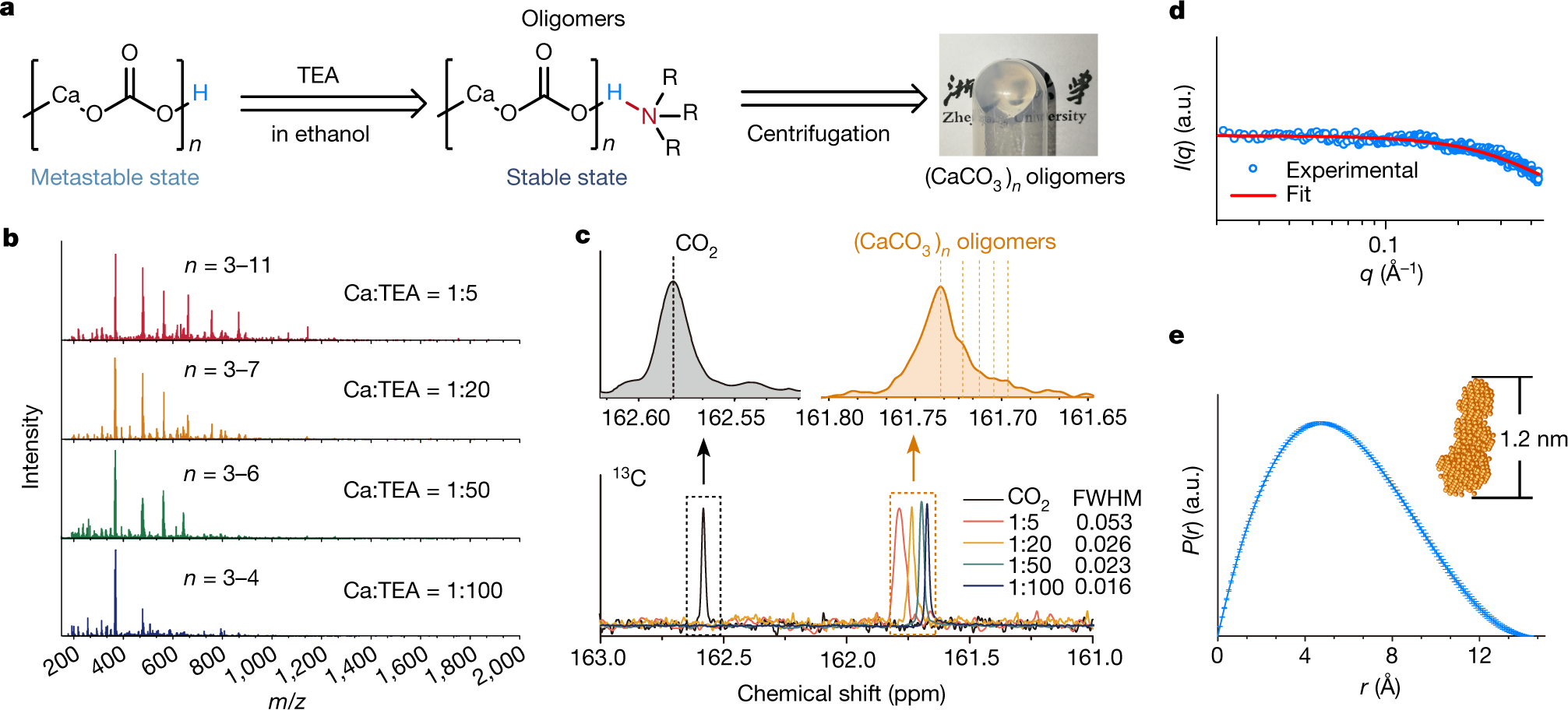
The caption:
a, Left, scheme of the capping strategy and reaction conditions for producing (CaCO3)n oligomers; right, a photograph of gel-like (CaCO3)n oligomers. b, Mass spectra of (CaCO3)n oligomers with different Ca:TEA molar ratios. c, Liquid-state 13C NMR spectra of CO2 or the carbonates of (CaCO3)n oligomers with different Ca:TEA molar ratios in ethanol. d, Scattering plots of (CaCO3)n measured by SAXS. The red curve is the fitting result obtained using DAMMIF. I, scattering intensity; q, scattering vector. The error bar represents the standard deviation of twenty measurements. e, Pair–distance distribution function (P(r)) of the (CaCO3)n oligomers. The inset shows the shape simulation of the oligomer. Error bars represent one standard deviation, n = 20.
The fate of the chloride ions is not reported. This seems to me to be an important consideration, nevertheless this is an interesting paper.
Cross linking of the polymers is achieved by evaporating the ethanol and the triethylamine.
Another graphic:
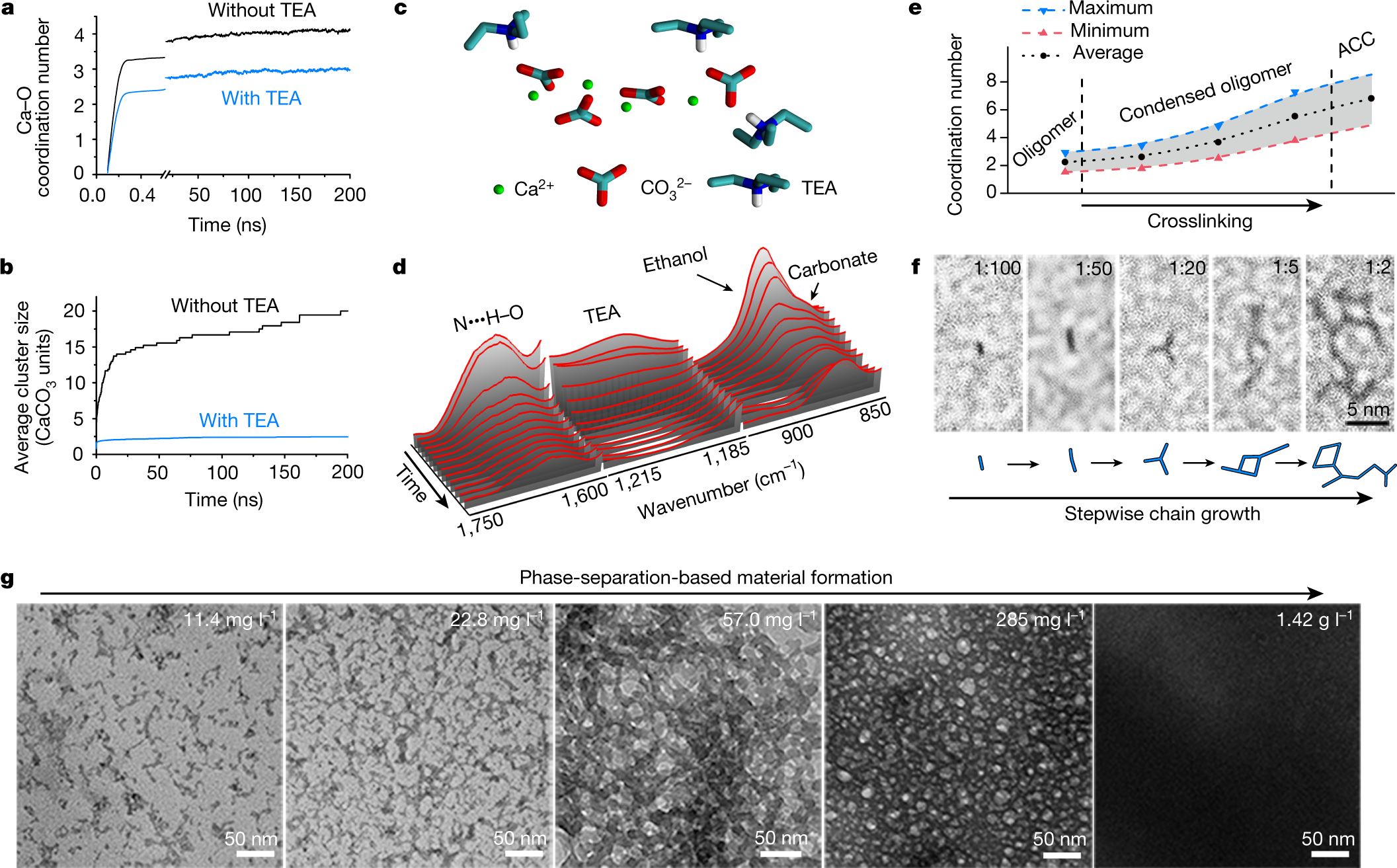
The caption:
a, b, Molecular dynamics simulation of the evolution of the Ca–O (from carbonate) coordination number (a) and the average cluster size (b) from ions (Ca2+ and CO32?

in the absence (black) or presence (blue) of TEA. c, A typical simulated CaCO3 cluster capped with TEA (an oligomer). d, In situ FTIR spectra during the drying of (CaCO3)n oligomers. e, The change in the coordination number of Ca–O during crosslinking. Owing to the uncertainty in the exact density during measurements, the blue and red lines are shown to represent the maximum and minimum coordination number of Ca–O, respectively. The black line shows the average coordination number. f, High-resolution TEM images of (CaCO3)n oligomers grown at different Ca:TEA ratios from 1:100 to 1:2. g, TEM images depicting the transformation of (CaCO3)n oligomers to larger structures during condensation.
Some text about the results:
Centimetre-sized monolithic CaCO3 materials were obtained by the crosslinking of oligomers. The resulting bulk maintained the original morphology of the compact gel precursor (Fig. 3a). FTIR spectroscopy and thermal gravimetric analysis indicated the formation of pure ACC without organic residue (Extended Data Fig. 7a, b). Scanning electron microscopy (SEM) and TEM showed the structural continuity in the bulk (Fig. 3b–e), and the internal continuous and integral textures were confirmed by artificially creating a crack (Fig. 3c). At scales from nano- to micrometres, the fabricated material was fully dense and smooth with no porosity or cracks (Fig. 3d, e). A nanoindentation test revealed that the ACC sample (Fig. 3l, blue circle in Fig. 3m) had a Young’s modulus of 8.0 ± 1.6 GPa and a hardness of 0.33 ± 0.07 GPa; these values are greater than those of most plastic materials28.
Another picture:
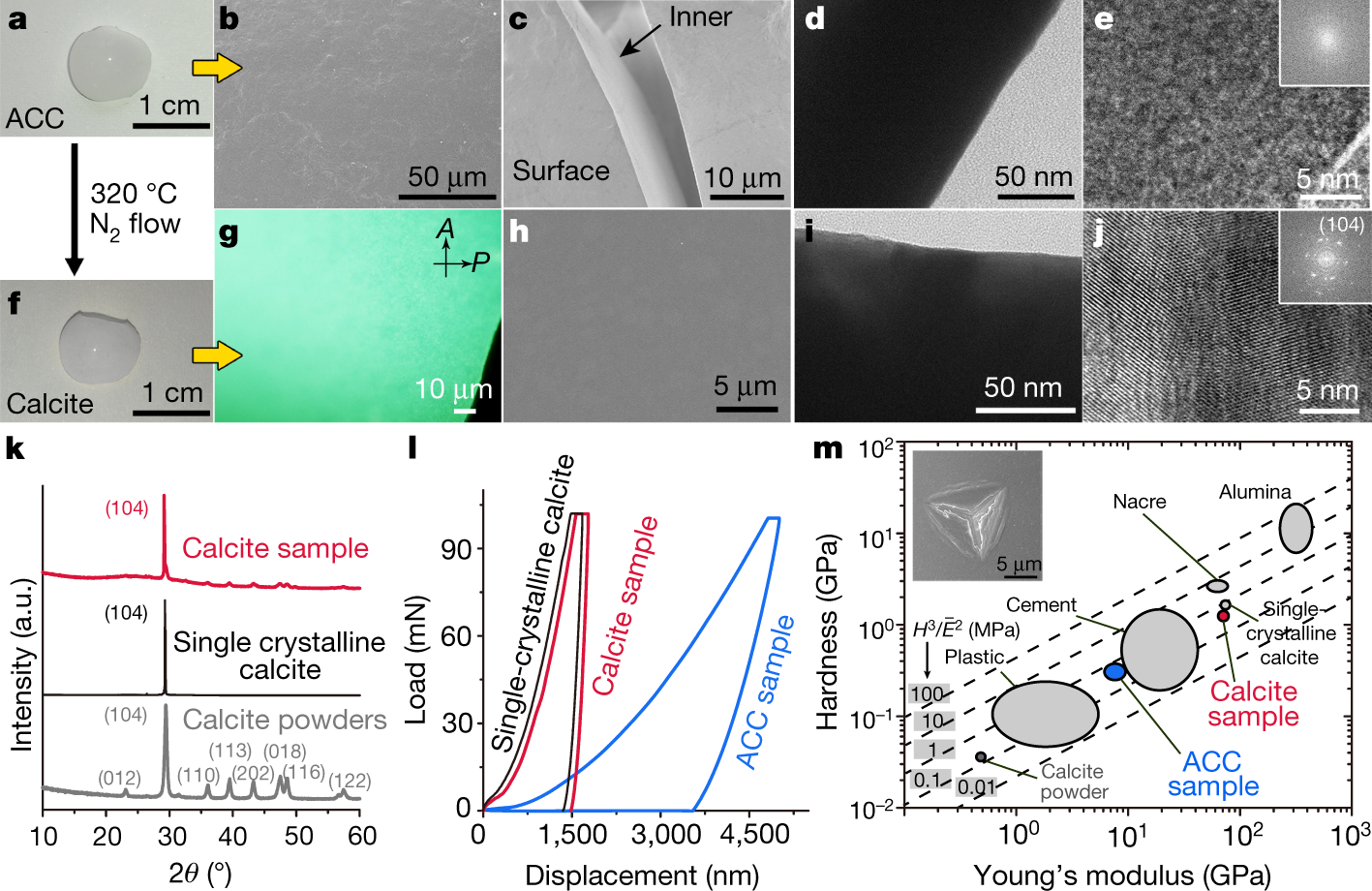
The caption:
a, Photograph of monolithic ACC prepared from (CaCO3)n oligomers. b–e, SEM (b, c) and TEM (d, e) images indicating the continuous solid phase of the prepared monolithic ACC. The inset of e is the fast-Fourier-transform image of the sample. Typically, the image of a crack in monolithic ACC exhibits continuity from the surface to the bulk (c). f, Snapshot of monolithic calcite prepared from monolithic ACC. g, Polarized-light optical microscopy (POM) image of the prepared monolithic calcite. h, SEM image of a surface on crystallized monolithic CaCO3. i, j, TEM images of the inner bulk of crystallized monolithic CaCO3. The inset of j is the fast-Fourier-transform image of the sample. k, XRD pattern of calcite powder, geological single-crystalline calcite and the calcite sample produced from (CaCO3)n oligomers. l, Load–displacement curves of the ACC sample, calcite sample and geological single-crystalline calcite sample measured by nanoindentation. m, Ashby plot of hardness (H) against Young’s modulus (E) for the prepared CaCO3 (including ACC and calcite) and other materials. The upper left inset is an exemplary residual indent of the Berkovich diamond tip on the crystallized CaCO3. Ē, plane strain modulus.
ACC, is amorphous calcium carbonate.
Some discussion of possible applications:
A considerable advantage of the crosslinking of ionic oligomers is that the oligomeric precursors can be moulded into shapes to enable continuously structured construction (Fig. 4a–c). This in turn enables the engineering of single-crystalline materials, including additive manufacturing. The construction of calcite rod arrays by oligomer crosslinking demonstrates the practicality of the preparation of single-crystal materials with structural complexity (Fig. 4d, e). This method can even be extended to repair damaged single crystals. Calcite single-crystal surfaces in optical devices30 can be damaged by mechanical crashing, scratching or corrosion, which reduces their functional performance—in particular transmittance. However, (CaCO3)n oligomers can generate oriented calcite within nano- and micro-sized pits or ditches of the damaged calcites in order to recover their smooth surface (inset of Fig. 4d, f, g). The repaired region (Fig. 4h, i) had exactly the same crystalline phase and orientation as the bulk beneath (Fig. 4j). The images of the high-resolution lattice fringes from the calcite bulk to the repaired front (Fig. 4k) demonstrate continuous (104) facets without any break, confirming that the same crystalline structure was reproduced exactly
A graphic on engineering utilizing this technique:
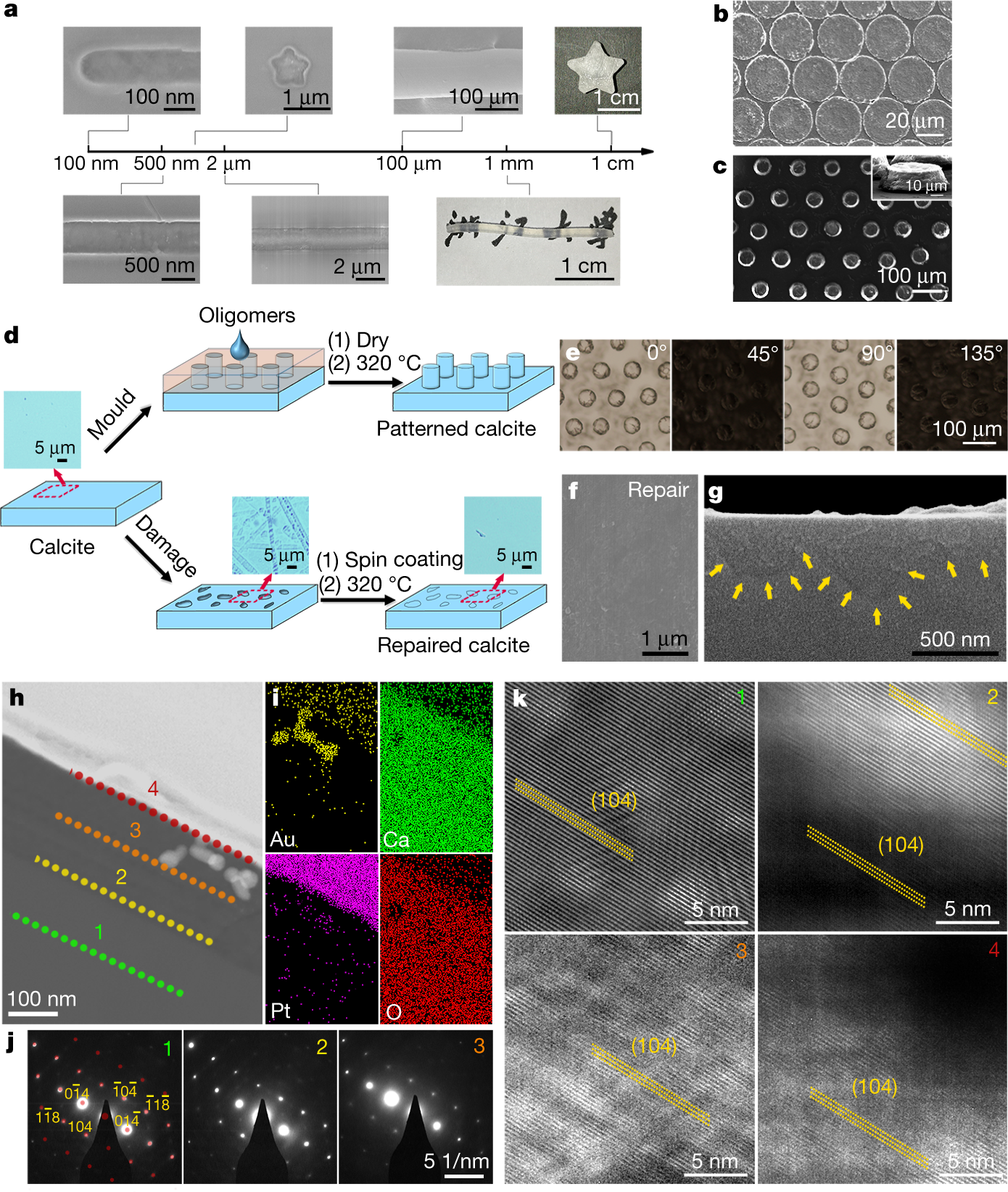
a, Moulded CaCO3 with different dimensions and morphologies. b, c, Moulded CaCO3 with different patterns. The inset of c shows a single CaCO3 rod. d, Schemes for pattern construction on single-crystalline calcite (top path), and the repair of rough single-crystalline calcite to smooth calcite (bottom path). The insets show optical microscopy images of the calcite surface at different stages: native, corroded, and repaired. e, POM images of the patterned calcite rotated at different angles. f, g, SEM images of the repaired calcite (surface and cross-section, respectively). h, TEM image of a cross-sectional view of the repaired calcite. The different layers labelled 1, 2, 3 and 4 were characterized by selected area electron diffraction and high-resolution lattice fringes in j and k. i, EDS mapping of the repaired calcite in h, showing the repaired CaCO3 as well as gold nanolabels. j, Selected area electron diffraction patterns of different layers (1–3) of h with an aperture of around 170 nm in diameter, showing the same patterns from the bulk to the repaired surface. The red dots in 1 are the simulated diffraction pattern viewed along the <?4, 4, 1> zone axis. k, High-resolution lattice fringes at the different layers (1–4) of h, exhibiting the facets of (104) with exactly the same orientation from the bulk to the repaired surface.
Interesting paper, I think, I thought I'd share it.
Have a great Friday.



