Science
Related: About this forumMolecular tuning of CO2-to-ethylene conversion
The paper I'll discuss is this one: Molecular tuning of CO2-to-ethylene conversion. (Sargent et al, Nature volume 577, pages 509–513(2020))
In the case where carbon dioxide is reduced to make ethylene, the starting material for polyethylene and other polymers, the carbon so obtained is sequestered.
As I noted in a previous post, plastics have their own risks, huge risks in a purely environmental sense, but long term use of plastics as opposed to single use, will sequester carbon dioxide. Nothing is ever black and white.
The paper's abstract begins with the usual paean to the regrettably popular "renewable energy" Gods, although all the worship focused on them has not made them to do anything at all to address the rapidly rising use of dangerous fossil fuels, coupled intimately with the rapidly rising rate of the destruction of the planetary atmosphere. This said, one of the limitations of and reason for the failure of the so called "renewable energy" industry in addressing climate change is that it produces electricity at times that it is not needed, making the electricity generated have no value whatsoever while destroying the value of electricity produced by reliable sources, thus driving overall electricity prices up, not down. (There is a reason that Germany and Denmark have the highest consumer electricity prices in the OECD.)
Everybody loses when electricity prices go negative despite rhetoric to the contrary.
To address fluctuations in demand, however, most power grids maintain generation that is not utilized and not sold: This is termed "spinning reserve" and is designed to address demand surges. In a sensible world, as opposed to the world in which we actually live, "spinning reserve" might actually be utilized for production purposes with a capability to be rapidly switched to a grid when needed. This would tend to reduce electricity prices, since it would allow for the utilization of continuous reliable baseload power systems such as those produced by nuclear energy, which is measurably the most reliable system of electricity production in the world and also the cleanest, to produce value at all times.
Thus this paper is interesting.
From the text:
These pyridinium type systems build on the work of Emily Cole and Andrew Bocarsly at Princeton University from some years ago. (Dr. Cole's company built on this technology failed however.) Their work is referenced in this paper. I'm glad to see this. I met Dr. Cole once, and I liked her very much.
Here are the structures of the pyridinium systems utilized in the paper:
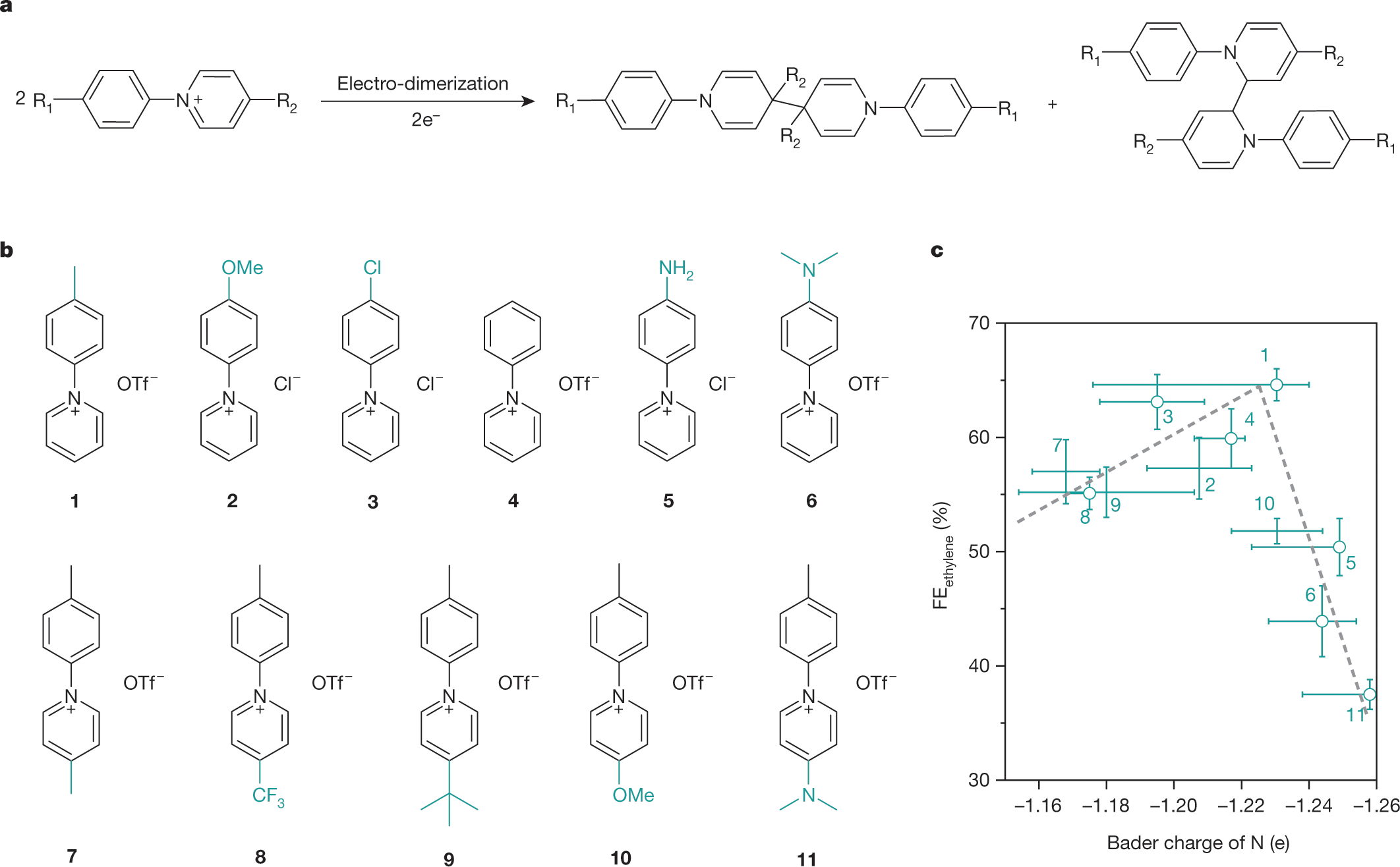
The caption:
The authors continue:
We evaluated CO2RR properties of these tetrahydro-bipyridine-functionalized electrodes in a liquid-electrolyte flow cell system (Supplementary Fig. 11), using CO2-saturated 1 M aqueous KHCO3 as the supporting electrolyte. In this system, the abundant catalyst/electrolyte/CO2 triple-phase interfaces overcome the CO2 mass-transport limit17,18 and thus enable commercially relevant current densities19,20. We note that, although the large achievable current densities in the flow cell drive up local pH (Supplementary Fig. 12), the tetrahydro-bipyridine layer does not create a further pH gradient near the active Cu surface (Supplementary Note 2).
The authors synthesized a chemical library of pyridinium salts to test, a good idea. Chemical libraries have come a long way since the early days, days in which I personally had occasion to work on them.
A few more graphics:
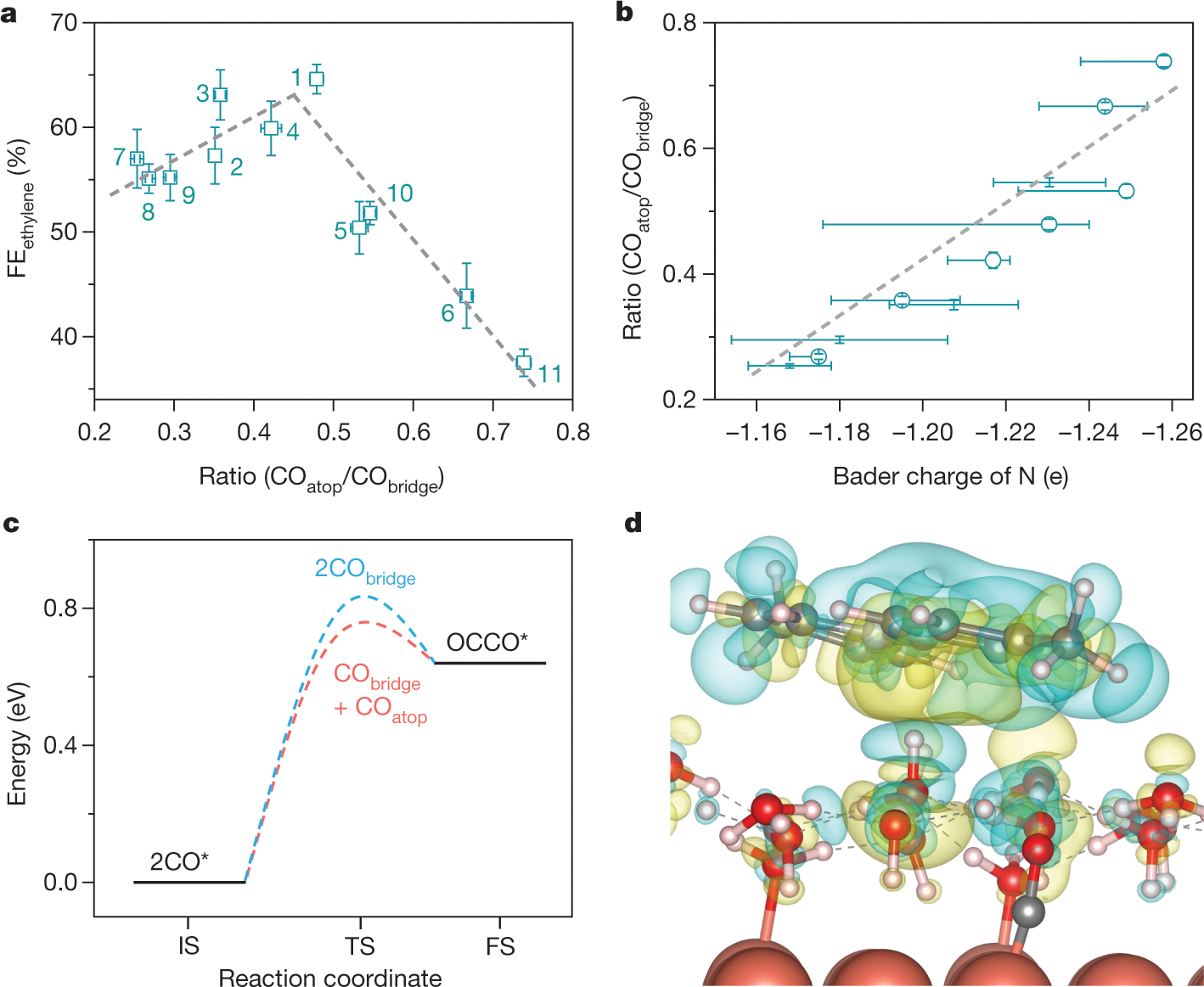
The caption:
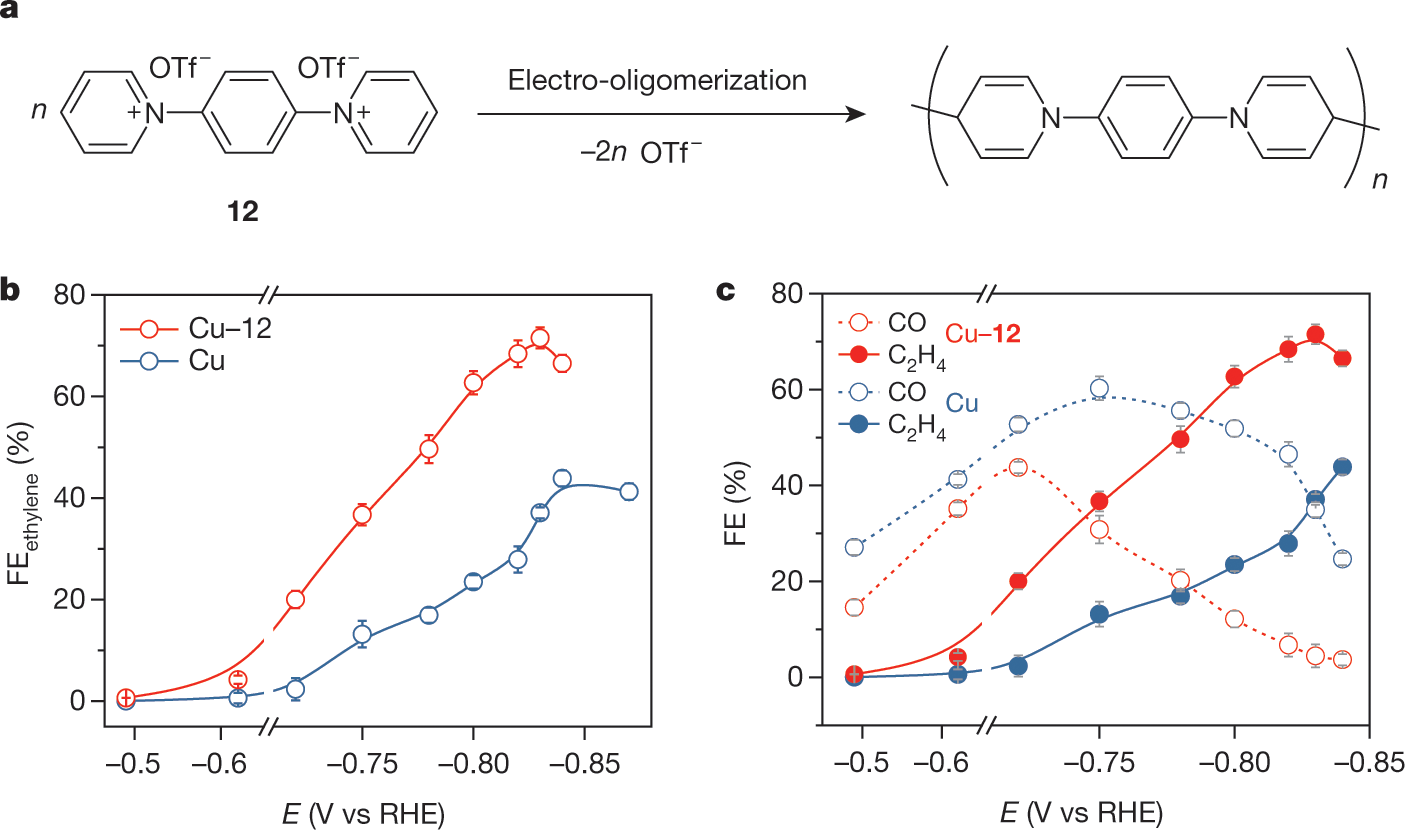
The caption:
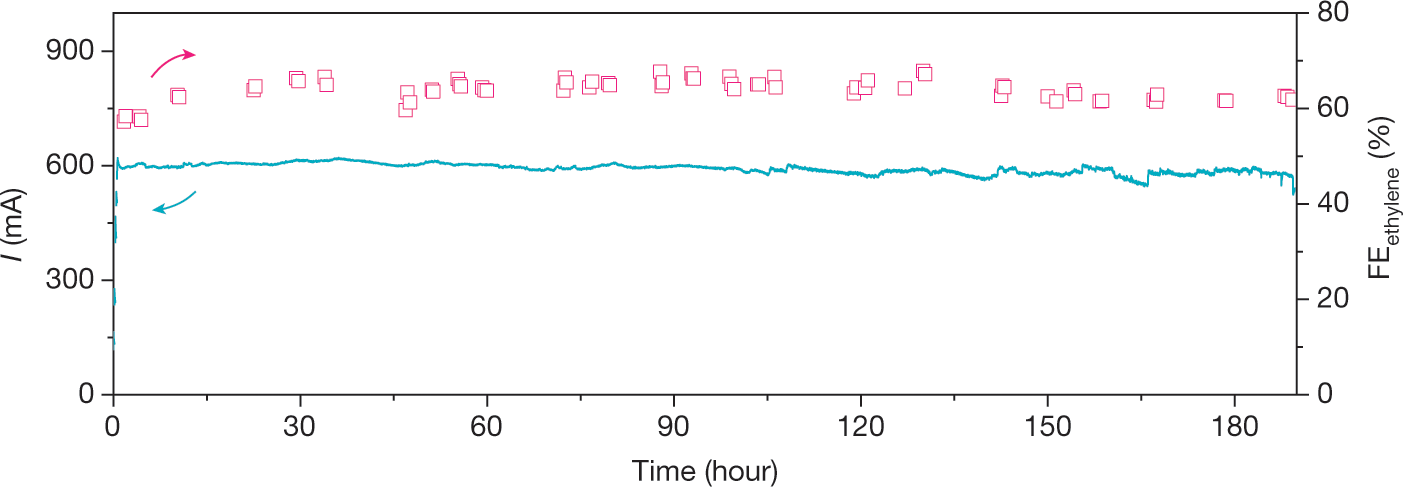
The caption:
The authors conclusion:
Cool if it works, although as energy storage, it is not particularly efficient, even less efficient than all those tons of batteries on which people want to foolishly bet the future of the planet.
I hope you're having a nice Saturday afternoon.
pbmus
(12,422 posts)(There is a reason that Germany and Denmark have the highest consumer electricity prices in the OECD.) ![]()
Another reason is Russian oligarchs who own gas companies that Germany and Denmark use for electricity production...
NNadir
(33,474 posts)The entire output of all the wind turbines in Denmark is roughly the equivalent of two or three dangerous fossil fuel plants.
Denmark, in case you're not aware of it, is an offshore oil and gas drilling hellhole. They sell gas to Germany, as do the Russians.
If I was selling gas, I'd be very fond of so called "renewable energy." Nothing entrenches the fossil fuel industry quite so well as the lipstick on the pig, so called "renewable energy," which has been, is, and always will be an expensive, trivial and useless form of energy.
Of course, if was selling gas, I'd be killing the future of all humanity, wouldn't I?
For the record, Charles Forsberg of MIT has published an outstanding study of the economic cost of so called "renewable energy" by comparing load rates and times of peak production of renewable energy. He notes what is often overlooked by dumb people crowing about negative electricity prices, which is that when electricity prices are negative, as happens on occasion in gas dependent hellholes like Germany and Denmark, the people who own wind turbines are receiving a zero return on their putative "investment."
(Charles Forsberg is nowhere near as hostile to so called "renewable energy" as I am, for the record.)
I have also posted some data in this space showing that even in California, the peak electricity demand comes about six to seven hours after noon time. So much for the silly "solar will save us" meme.
We've been crowing about so called "renewable energy" for half a century, almost my entire adult life. Two days ago we hit 414.08 ppm of the dangerous fossil fuel waste in the planetary atmosphere, roughly 100 ppm more than I was born.
We just don't get it.
pbmus
(12,422 posts)NNadir
(33,474 posts)...a US Navy Researcher, Heather Willauer has already developed a technology that utilizes the carbonates in seawater, carbonates formed by the absorption of air CO2 to make jet fuel. The technology makes fuel at a cost of around six dollars a gallon, which turns out to be economic in very remote regions, for example, at the US Naval base in Diego Garcia.
Nevertheless, the technology represents a huge engineering challenge and, - I don't think people really appreciate this, nor are there journalists who, in my opinion, who can appreciate this - a huge energy penalty for doing it, because not only have we dumped carbon dioxide on all future generations, but we have also dumped entropy on them.
Nevertheless, with cheap nuclear energy it is feasible, but it is not simple.