Science
Related: About this forumThe Effect of Pressure on Enhancing Covalent Bonding in Curium Complexes.
The paper I'll discuss in this post is this one: Compression of curium pyrrolidine-dithiocarbamate enhances covalency (Eva Zurek et al., Nature 583, pages 396–399(2020))
Sometime ago, in this space, I wrote about the critical masses of several americium isotopes: Critical Masses of the Three Accessible Americium Isotopes.
Perhaps in a smarter world than the one in which we live, the potentially very valuable properties of americium based nuclear fuels will be recognized, only one of which will be as a source of what is sure to be, in the not so distant future, a very, very, very rare yet useful gas, helium, which our generation is pissing away in typical disregard for all who come after us. (The technological importance of helium, seldom appreciated, far outstrips its importance in children's balloons.)
Whatever.
Because of the high energy to mass ratio of nuclear fuels - the very thing that makes them environmentally superior to all other forms of primary energy - the amount of helium produced by americium fueled reactors will be relatively small, but in the case where prices rise to extremes because of scarcity, may have significant economic import. Although Americium does release helium in its normal decay, in the case of Am-241 to neptunium, and in the case of Am-243 to plutonium, the long half-lives of these elements prevents this element from ever becoming an important source of helium when relying on ordinary nuclear decay. However as a nuclear fuel, necessarily in a fast neutron spectrum, owing to the high capture to fission ratio, significant quantities of helium will be produced from the nuclear decay of curium, specifically curium's isotopes 242 and 244, as well as the decay daughter of 242, Pu-238.
Thus the chemistry of curium is a worthy study, although one doesn't see as many explorations of curium chemistry as perhaps one would like.
Curium is a relatively pedestrian element in comparison to the earlier actinides, nothing like the wild and woolly chemistry of plutonium for example, and it is the first element among that actinides that behaves very much like an lanthanide in most respects, dominated by the 3+ oxidation state. The abstract, which should be open, describes why this is succinctly:
...but the abstract continues...
Normal atmospheric pressure is about 100 kilopascals, so we're talking pressures that are thousands of times atmospheric pressure.
The authors worked with Cm-248 which is of (relatively) lower radioactivity owing to its very long half life, 348,000 years, as well as the even lower radioactivity of its daughter, plutonium-244, half-life 80 million years. Plutonium-244 is considered an extinct nuclei which most likely survived long enough to accrete with the earth; the existence of a xenon isotope in our atmosphere is believed to have resulted from the spontaneous fission of this isotope. (A few atoms of possibly primordial Pu-244 have reportedly identified in very old lanthanide ores in California.) There is also a curium isotope that is considered to be an extinct nuclide, Cm-247, which has a half-life of around 15.6 million years, but it is more difficult to obtain because of its high fission cross section, and in any case, its decay daughter Pu-243 is very radioactive, and leads to the fairly radioactive daughter Am-243.
Anyway, the authors made a pyrollidine thiocarbamate complex of curium to study the effects of pressure.
A picture:
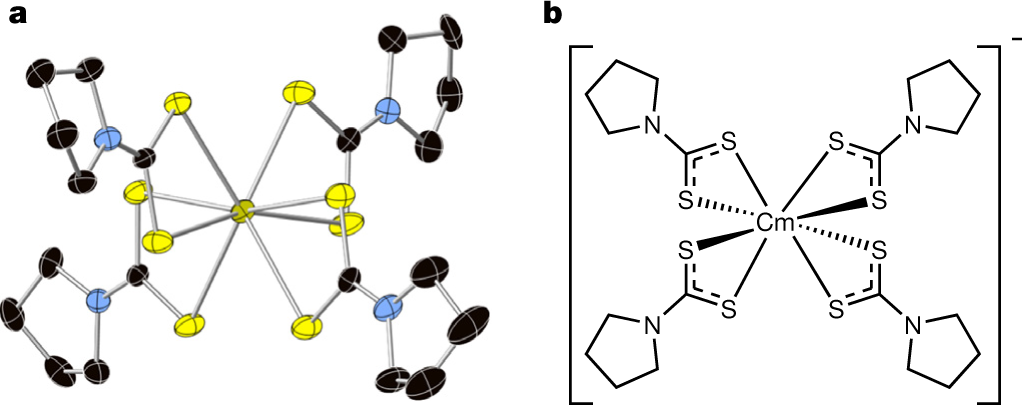
The caption:
They then conducted spectral analysis of the ammonium salt of this complex at various pressures:
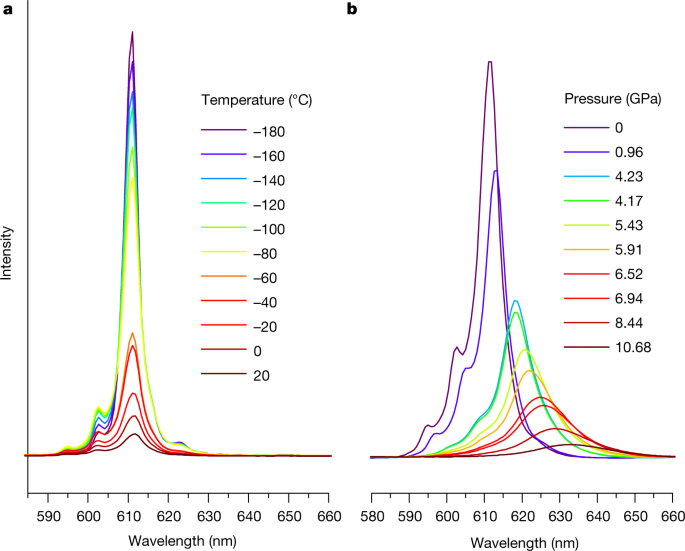
The caption:
"Stark Levels" by the way, were discovered by Nobel Laureate Johannes Stark. There was on the shelf in Princeton's Firestone library in German written by him, a fascinating book through which I leafed one time - it's now been carried off to the Recap, and one would be embarrassed to request it. The guy would be right up Trump's ally except for the fact that Stark was a scientist, and Trump despises science, inasmuch as the book I found by Stark was written in the early 1930's and was all about how wonderful Adolf Hitler would be for German science, proving definitively that a criterion for winning the Nobel Prize does not generally include any requirement for possessing character.
Hitler destroyed German science, and Stark, a notably anti-semite, was arrested after World War II and subject to de-Nazification steps, whatever de-Nazification involved. I doubt he could be reformed any more than Bill Barr or Steven Miller or Donald Trump could be "De-nazified."
Sorry, I didn't mean to divert from the scientific subject.
Another complex of curium utilized is a metillate complex, that is a salt of benzene hexacarboxylic acid. It's called "Cm-2."
Here's a graphic comparing the pressure effects of the two complexes.
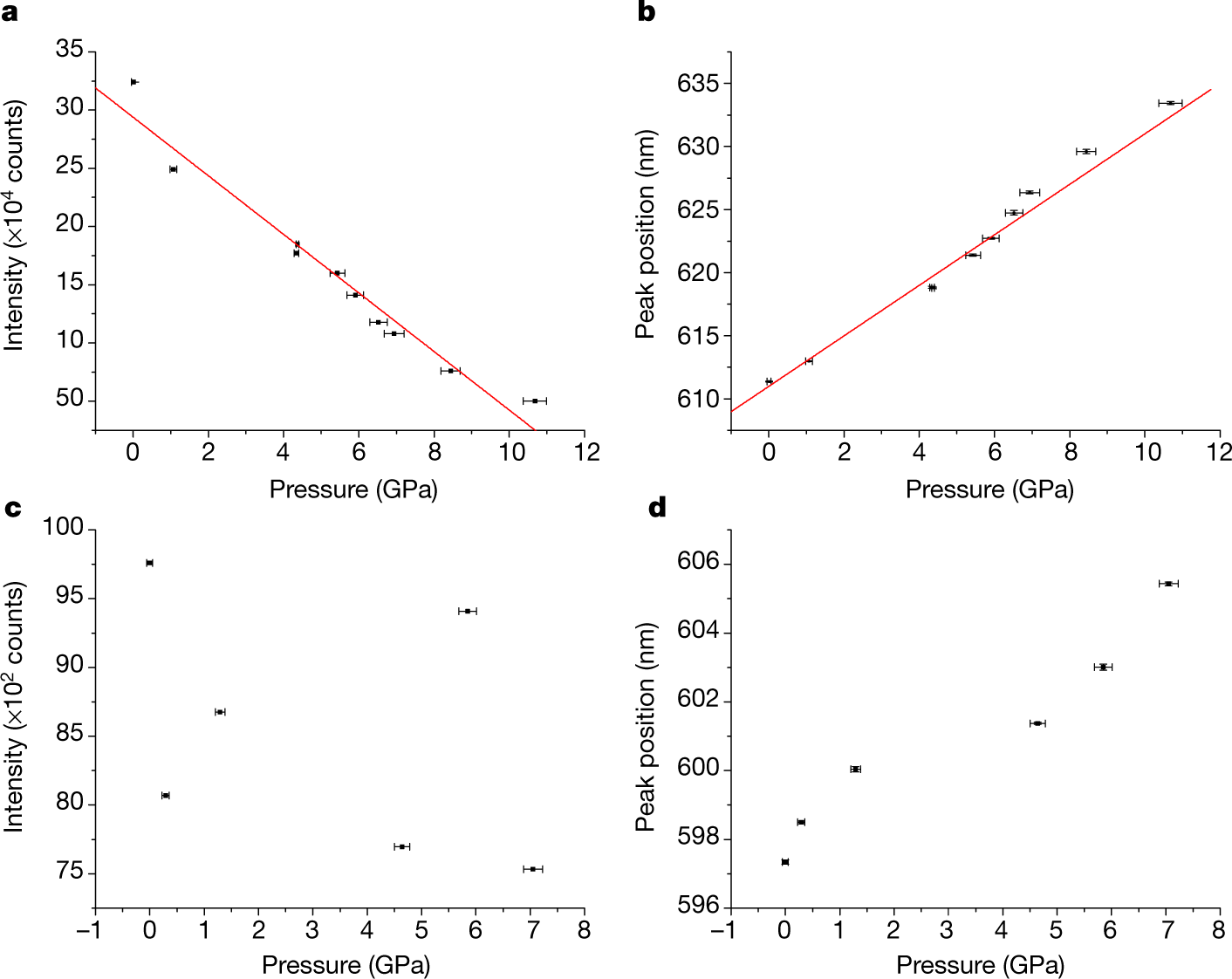
The caption:
The authors perform some in silico (DFT) analysis to generate this molecular orbital cartoon:
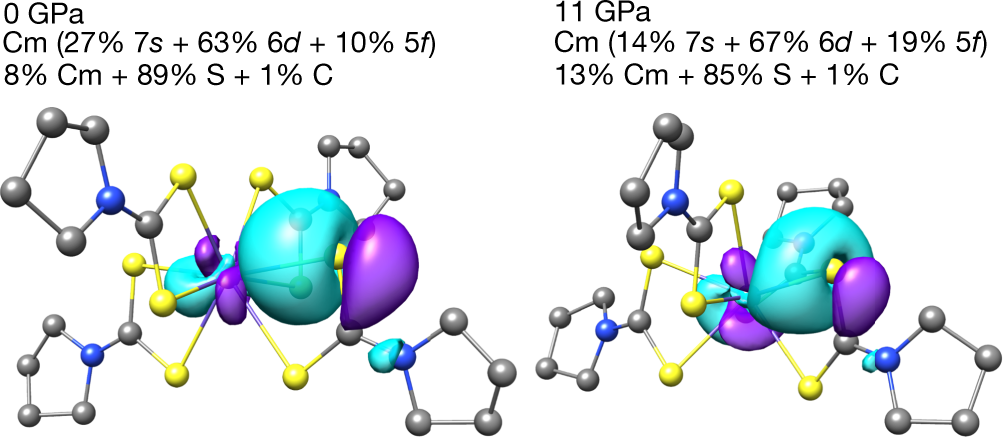
The authors conclude thus:
How much practical import this work might have I can't say. It may be true that pressure changes may represent a tool in the difficult separation of curium from the lanthanides, allowing for the recovery and use of lanthanide fission products in the presence of large amounts of curium. Pressure plays an increasing role in separations chemistry, and it's conceivable at least, that this work may show the way.
Such separations may well be increasingly important if we are to save the world using nuclear energy.
I've been thinking about such separations quite a bit lately. I started to write a post about some papers I read on the separation of xenon and krypton - both of which are also fission products - which kind of caused my head to explode, and sent me off on tangents about chemistry about which I haven't thought in a long time, and even some I never knew. As I age, I rarely experience the emotions connected with an entirely new way of thinking about things, and so I haven't found time to write here all that much, since this rare thing happened, not that anything I write is of all that much general interest. I do learn things however when I write these posts, and that I suppose, is the selfish point.
Anyway. In spite of Covid, I hope you are managing to enjoy the summer season and the growing realization of how lucky we are to be alive in amazing, if hard, times.
hunter
(38,302 posts)--more--
https://recap.princeton.edu/
NNadir
(33,470 posts)...Collections.
When you search on the Princeton Library search engine, it simply says "Recap," and you have to order it.
I've requested books from ReCap quite often, since they have all this obscure stuff about nuclear reactor technology from the 1950's and 1960's when creativity in nuclear engineering was still allowed and not subject to scare stories by people who have no knowledge of anything.
In the 1950's and 1960's as opposed to now, the Princeton Community was not hostile to nuclear energy and hadn't drunk the "renewable energy will save us" Kool Aid, so the collection is quite rich. Things I've found there include, among other thrilling stuff, include photographs of an actual three dimensional model, 4 dimensional actually, which had ternary phase diagrams superimposed on a temperature (z-axis) of actinides.
One can also find obscure stuff about the origins of the anti-nuke movement, which actually had its roots in a very wealthy area of Long Island, Lloyd's neck, when LILCO threatened to build a nuclear plant among the estates.
Of course, I didn't know that some of what is in ReCap is actually from other universities.
By my estimate, at least 200 million people have died from air pollution as a result of this attempt to "pull on Superman's cape," by placing industrial infrastructure near rich people. LILCO was very, very, very, very bad at promoting nuclear energy, despite the good intentions of their forward looking engineers. I have a certain sympathy for those guys, Long Island engineers in the 1950's, since they had no way of imagining the power of ignorance and the power of stupidity. It was a time that science was honored.
The power plants on Long Island today burn trash and they burn gas, and as a result, the island is sure to go largely underwater in this century. It nearly did so in Hurricane Sandy.
The Princeton Library is closed now; and for the first time in nearly 20 years, I'm not going there several days a week. It's very, very, very painful. I do have a ton of reading on which I can catch up before the re-opening, should I live long enough to experience it, but my access to the latest and greatest is diminished; not gone, but diminished.
In these times, the triumph of ignorance is palpable.
eppur_se_muova
(36,247 posts)There were so many recognizably German names I expected this to be an all-European group. But three of the correspondents are at American universities. Postdocs, or what ? Just wondering about the state of American science institutions vs the rest of the world in the era of IQ45.
NNadir
(33,470 posts)It's rather disturbing that the Actinide science facility for Europe is located in Germany. at Karlsruhe.
A lot of very good papers have come out of there.
Germany is, of course, phasing out nuclear energy and becoming dependent on gas as is fashionable these days.
That's unfortunate for all future generations; and the destruction of German nuclear infrastructure will be hard to reverse.
I used to read a lot of Calphad papers out of there; although I'm not quite sure how accurate these calculate phase diagrams actually are, and how subject to experimental verification.
I just came across a great monograph out of India on the experimental determination of liquid actinide phase diagrams, so the centers of knowledge will move elsewhere.
American science is of course, much as was the case in 1930's in Germany, under attack by superbly ignorant politicians.
It actually took Hitler - who was much more of a magnetic, if malign, character than the orange carny barker in the White House and his enablers in Congress and in the Governor's mansions down your way - a fairly long time to totally destroy German science. Lise Meitner was actually able to work in Germany until 1938, and some German scientists actually survived in Germany until 1945.
(All of us in the Science forum hope for your health and safety, by the way, since you are in the region of official Covid denial.)
There is that famous story - possibly apocryphal - of David Hilbert's conversation with the German Minister of "Education" - Nazi Germany's Betsy DeVos - whereupon Rust asked how mathematics was thriving at Göttingen now that "Jewish influence" had been removed, to which Hilbert allegedly replied in effect, "there is no mathematics at Göttingen."
American science is on the ropes, but one hopes that because of our cultural traditions of democracy, there may yet be time to save it.
I never imagined Nazis running our government, but complacency was certainly ill advised. I do feel the American people rising, and am cautiously optimistic, but great damage has been done, as in so many other areas of our country, to American science.
May we avoid the fate of Germany.
By the way, some of the authors of this paper did train in Germany, if I recall correctly, at Karlsruhe.
eppur_se_muova
(36,247 posts)Like a couple of other Red Governors, she has seen the virtue of bucking the carny barker, even if only quietly.
We had a county-wide mask order in place before that. We have been living all along as if an order were in place, since about Feb. Thank you for thinking of us.
Interesting to describe a Nazi Minister as their DeVos, instead of DeVos as our version of a Nazi minister. The former now starts to seem like the worse insult, does it not ?