Science
Related: About this forumPlutonium-244 Discovered on the Ocean Floor Constrain the Yields of Recent Local Supernovae.
Last edited Thu Nov 25, 2021, 10:11 AM - Edit history (1)
The paper I'll discuss in this post is this one: 60Fe and 244Pu deposited on Earth constrain the r-process yields of recent nearby supernovae (Wallner et al., Science, 14 May 2021: Vol. 372, Issue 6543, pp. 742-745)
Not so long ago in this space, I referred to the campaign taking many years to produce the world supply, 7 grams, of the very long lived isotope of plutonium, Pu-244: Recovery of Trivalent Lanthanides and Transplutonium Actinides with Resin Supported Diglycomides. This rare isotope has a half-life of 81.1 million years, making it useful as an internal standard in nuclear forensics, as a target for the synthesis of the super-heavy elements, and for the study of chemical properties of the element, while minimizing occlusive effects associated with the higher radioactivity of isotopes with a longer half life. Very little Pu-244 is formed in commercial nuclear reactors, nor were large amounts formed in the era of nuclear testing. Early reports indicated that Am-244 had a branch ratio by which a tiny amount, on the order of 1 decay in 1000, decayed by electron capture to give Pu-244, but when I went to the latest data tables at the Brookhaven National Laboratory's Nuclear Data pages, it is now reported that this isotope actually decays 100% by ?- to give Cm-244. This means that the only path to the creation of Pu-244 relies in neutron capture in Pu-243, an isotope with a short half-life, about 5 hours, or by the decay of Cm-248, which can only be produced with very long irradiation times, and in any case, has a long lifetime - its half-life is 348,000 years - precluding it's use as a Pu-244 source. Although I do not know whether the fission cross section or capture cross sections of Pu-243 are known, as a nuclei with an odd mass number and an even atomic number, the fission to capture ratio is likely to be fairly high. Nevertheless, it is possible, as it was in historical high flux research nuclear reactors, to produce rather small amounts of this isotope. (It is also believed that small amounts of Pu-244 may have been formed in historic open air hydrogen bomb tests, which produce high neutron fluxes, but the isotope has not been detected in fall out to my knowledge.)
The situation is quite different in collapsing stars, supernovae, where the neutron flux can easily dwarf anything that can be generated on Earth, as well as during collisions between neutron stars. The collapse of these stars has produced all of the uranium and thorium, the billions of tons of each, that now drives the internal heat of the Earth (with a minor assist from radioactive potassium-40). This takes place during the "r-process" in which nuclei absorb neutrons faster than they can decay by beta emission, thus producing very heavy nuclei up to an including fermium, and perhaps even higher. As a result, much of the thorium-232, the isotope that dominates all of Earth's thorium, may be the decay product of Pu-244 and/or it's precursor, Cm-248.
The long half-life of Pu-244 suggests that may have survived long enough to have accreted in the early Earth, and it believed that an isotope of the xenon present in the Earth's atmosphere may have been formed by spontaneous fission of this historic Pu-244. One can calculate that 1 kg of Pu-244 in the early Earth would leave behind about 48 million atoms after 4.5 billion years.
In 1971, the great nuclear chemist Darleane Hoffman reported that she had discovered a few atoms of Pu-244 in lanthanide ores at the Mountain Pass mine, after reasoning that the geochemistry of plutonium should be very similar to that of cerium and thorium, both of which are constituents of these ores. (Thorium is a by product of the lanthanide ("rare earth" ) mining on which so called "renewable energy" and many other technologies depend. If recovered from mine tailings, this thorium could in theory replace all mining for energy purposes for many hundreds of years.) Her report, which has not been confirmed by the use of accelerator mass spec, is here: Detection of Plutonium-244 in Nature (D. C. Hoffman, F. O. Lawrence, J. L. Mewherter & F. M. Rourke, Nature volume 234, pages132–134 (1971))
The paper cited at the outset refers to Pu-244 that is believed to have rained down on Earth in recent times as a result of supernovae that took place near our solar system in the last tens of millions of years.
The abstract is open sourced. From the introduction to the paper itself:
The Solar System (SS) is located inside a large ISM structure [the Local Superbubble (LB)] that was shaped by supernova (SN) explosions during the last ~12 million years (Myr) (8). Earth has therefore been exposed to both ejecta from the SNe and swept-up interstellar material that traversed the SS during this time period (9, 10). Dust particles from the ISM pass through the SS (11) and contain nucleosynthetic products of stellar events (e.g., stellar winds and SNe) (10, 12, 13). Earth’s initial abundance of the 60Fe radionuclide [half-life (t1/2) = 2.6 Myr (14, 15)] has decayed to extinction over the 4.6 billion years (Gyr) since the SS’s formation. 60Fe, however, is produced in massive stars and ejected in SN explosions. Evidence for the deposition of extraterrestrial 60Fe on Earth has been found in deep-sea geological archives dated to between 1.7 and 3.2 million years ago (Ma) (16–20), at recent times (21, 22), and possibly also around 7 Ma (19). 60Fe has also been detected in lunar samples (23), in astronomical observations of gamma rays associated with its radioactive decay (24), and in galactic cosmic rays (25). SN activity in the last ~2 Myr is suggested by an excess in the local cosmic-ray spectrum (26). Other radionuclides are also produced and ejected in such explosions (9, 27–30). If substantial r-process nuclei are produced in SNe this would also have enriched the local ISM with actinides, such as 244Pu. With a half-life of 80.6 Myr, 244Pu is much longer lived than 60Fe, so it can be contributed by older r-process events, not limited to those that formed the LB. Either as part of the SN direct ejecta or as continuous ISM influx, we expect dust particles containing 244Pu to enter the SS, similarly to 60Fe, but probing different nucleosynthetic processes. Previous measurements in terrestrial or lunar archives have provided only upper limits on actinide influx (12, 31–33).
We searched for extraterrestrial 60Fe and 244Pu incorporated into a deep-sea sample on Earth—a ferromanganese crust (which we refer to as Crust-3) that spans the last 10 Myr, sampled at ~1500 m below sea level in the Pacific Ocean, with 115-cm2 cross-sectional area and ~25-mm thickness (27). The radionuclides were identified and counted using accelerator mass spectrometry (AMS) (27). For 60Fe, a time-resolved depth profile of ~1-cm2 area was analyzed, subdivided into 24 layers, each ~1-mm thick, corresponding to a time resolution of ~0.4 Myr per layer [crust growth-rate of ~2.4 mm Myr?1, dated with terrestrial 10Be (27)]. The remaining part of Crust-3 (114-cm2 area), after separating the aliquots used for 60Fe analysis, was split into three thick, horizontal layers designated 3/A (extending from 0 to 3 mm, equivalent to 0 to 1.3 Ma, with a mass of ~20 g), 3/B (3 to 10 mm, 1.3 to 4.6 Ma, 179 g), and 3/C (10 to 20 mm, 4.6 to 9.0 Ma, 208 g), given the anticipated low abundance of 244Pu. We expected the top layer to contain anthropogenic Pu from atmospheric nuclear weapons tests performed during the 20th century...
Radioactive iron-60 is also formed in nuclear weapons tests, from neutron capture in the steel casings, and it can also be formed in small amounts in commercial nuclear reactors having steel cores - this accounts for Co-60 in pressurized water and boiling water reactors. However, in sediments, the age of the sediments can be determined by examining the Co-60/Fe-60 ratio or the ratio of Ni-60, the final stable decay product. The authors here took other steps to differentiate the age of their sediments to differentiate them from nuclear weapons testing contamination. They used beryllium-10, the radioactive isotope that forms naturally in the Earth's atmosphere by spallation from oxygen and nitrogen interacting with cosmic rays.
They detected 435 atoms of Fe-60 in their samples.
Table 1 from the paper:
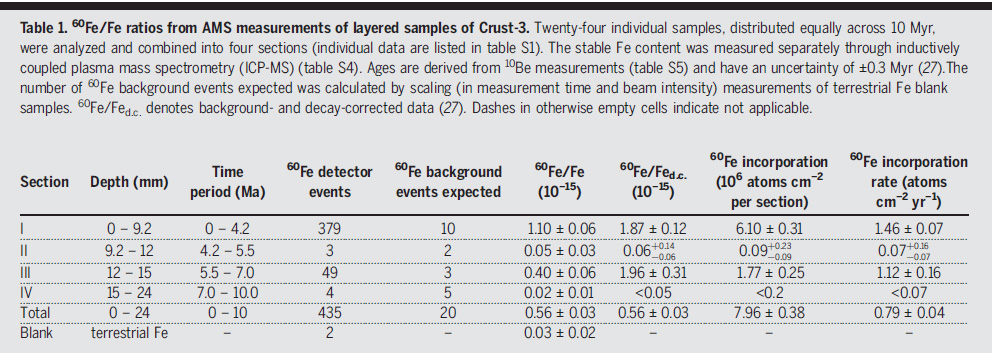
The caption to table 1:
Twenty-four individual samples, distributed equally across 10 Myr, were analyzed and combined into four sections (individual data are listed in table S1). The stable Fe content was measured separately through inductively coupled plasma mass spectrometry (ICP-MS) (table S4). Ages are derived from 10Be measurements (table S5) and have an uncertainty of ±0.3 Myr (27).The number of 60Fe background events expected was calculated by scaling (in measurement time and beam intensity) measurements of terrestrial Fe blank samples. 60Fe/Fed.c. denotes background- and decay-corrected data (27). Dashes in otherwise empty cells indicate not applicable.
They were also able to differentiate Pu-244 of extraterrestrial origin from nuclear testing contamination by determining the ratios of Pu-244 to its decay product Pu-240 and the presence or absence of Pu-239, Pu-241 (and its decay product Am-241), Pu-242:
Pictures from the text:

The caption:
(A) 60Fe incorporation rates for Crust-3. The data (red points) have been decay corrected, and each layer is equivalent to 400 thousand years. The absolute ages have an uncertainty of ~0.3 to 0.5 Myr (27). (B) 244Pu incorporation rates for the three layers after subtraction of the anthropogenic 244Pu fraction (27). (C) 244PuISM/60Fe number ratio in the crust sample with layers 1 and 2 combined (horizontal solid lines with shaded error bars). All error bars show 1? Poisson statistics.

The caption:
(A and B) Variations of the measured 240Pu/239Pu ratio (A) and the 244Pu/239Pu ratio (B) across the three layers (solid red lines). The dashed red lines and gray shading indicate 1? uncertainties. The blue shaded area and solid line represent the expected ratios for Pu from nuclear weapons fallout (27). 240Pu/239Pu remains constant across the three layers, whereas 244Pu/239Pu is enhanced in the deeper (older) layers. We attribute the excess above anthropogenic (anthr) levels to extraterrestrial 244Pu. Equivalent data for 241Pu/239Pu are shown in fig.
Table 2 from the paper:
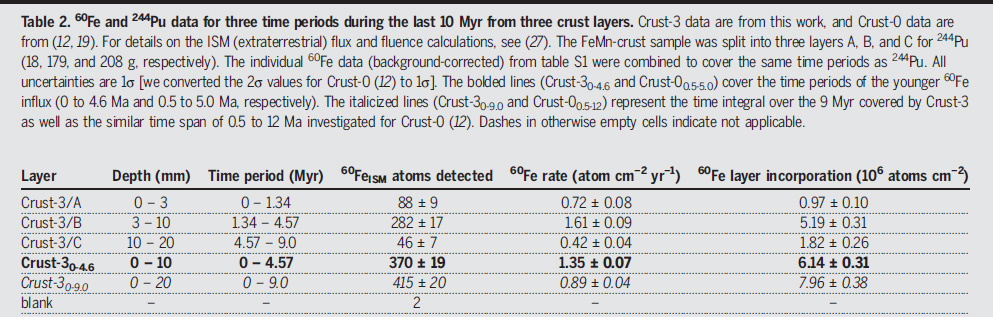
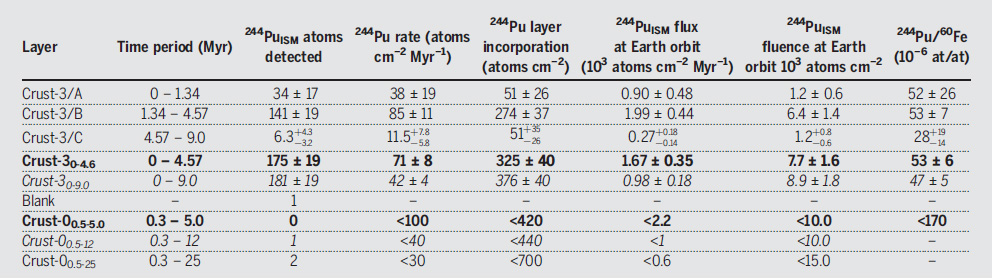
The caption to Table 2:
Crust-3 data are from this work, and Crust-0 data are from (12, 19). For details on the ISM (extraterrestrial) flux and fluence calculations, see (27). The FeMn-crust sample was split into three layers A, B, and C for 244Pu (18, 179, and 208 g, respectively). The individual 60Fe data (background-corrected) from table S1 were combined to cover the same time periods as 244Pu. All uncertainties are 1? [we converted the 2? values for Crust-0 (12) to 1?]. The bolded lines (Crust-30-4.6 and Crust-00.5-5.0) cover the time periods of the younger 60Fe influx (0 to 4.6 Ma and 0.5 to 5.0 Ma, respectively). The italicized lines (Crust-30-9.0 and Crust-00.5-12) represent the time integral over the 9 Myr covered by Crust-3 as well as the similar time span of 0.5 to 12 Ma investigated for Crust-0 (12). Dashes in otherwise empty cells indicate not applicable.
From the conclusion to the paper:
Esoteric, but cool, I think.
Have a great and safe weekend.
Best_man23
(4,897 posts)Need ![]() now, thanks for sharing.
now, thanks for sharing.
eppur_se_muova
(36,259 posts)
(For whatever obscure reason, my non-Vulcan brain read the first two occurrences of 'plutonium' as 'polonium' (!), so by the time I got to "half-life of 81.1 million years" I was starting to think I was losing my mind ! Relieved to see that on this particular occasion the conclusion is premature.)
I still have trouble grasping the idea that so much research nowadays is based on application of single-atom detection methods. It's one thing to detect single superheavy atoms spalling off of an irradiation target in hard vacuum, but to dig them out of natural samples just seems like wizardry still.
NNadir
(33,512 posts)...now.
These folks are using accelerator mass specs. You can now buy an FT-ICR mass spec with a 15 Tesla magnet for around a million and a half.
The interface between software, fabulous schemes and modes for ionization, ion manipulation and storage in traps, separations by cross section in ion mobility spectroscopy - there is now software that can immediately spit out the cross sectional area of an ion - and measurement of mass to charge ratios without collisions with physical contact between the ion and detector (orbitraps), it blows one's mind.
Sample prep used to be the big deal; now it's less and less necessary. People have spent lots of hours in the lab digesting proteins with enzymes like trypsin and chymotrypsin. Last week I heard of an electron dissociation device that can reproducibly break bonds to proline, doing the something very close to what enzymes do, in dirty samples with almost no separation.
Bruker has an interface to a 4000 core super computer that can search protein databases within minutes of completion of a sample run. I could be wrong, but the way I understood this, you just "phone it in," upload a *.wif file and it comes back and up on the screen.
It's all happening very fast. People used to spend their entire graduate careers elucidating the structure of glycans. Now it takes less than ten minutes, if that.
I am actually coming to believe that the time will come that even PCR will become obsolete, because mass spec will do it all.
It's amazing. When I was a kid, I never even thought about mass spec. You got one, you put it in the file. Everything for me was NMR. Now NMRs are beginning to seem just "cute."