NNadir
NNadir's JournalWill Data and Telecommunications Require 20% of the World's Electricity by 2030?
The news item I will discuss in this post is here: Nature 561, 163-166 (2018)
Here's the historic/soothsaying graphic that gets to the core of what is discussed therein:
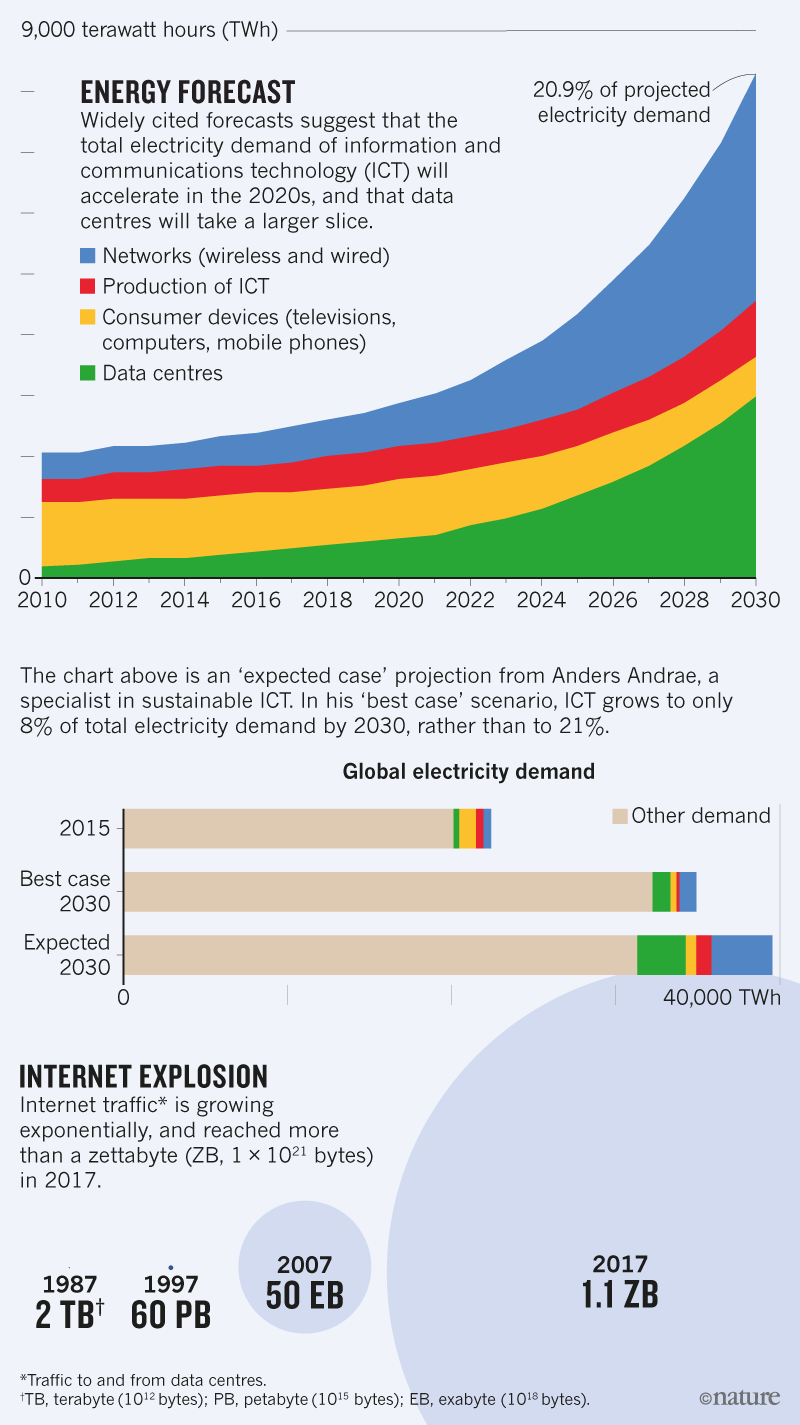
Some trademark acerbic commentary:
One of the fun things I like to do is to read predictions from 20 or 30 years ago about what energy consumption would be by now.
These come in three forms, serious analysis, hand waving, and pop wishful thinking, the latter usually with some breathless headline about a "study" - often from the innumerate clods at Greenpeace - that says that the world "could" run on "100% renewable energy by [insert year that the soothsaying writer will be dead here]"
The thing that characterizes these historical predictions which approaches 100%, particularly those in the "100% renewable energy by..." class, is that they were almost 100% wrong.
In the 21st century, the fastest growing source of energy (as opposed to peak power available for a few minutes a day on a good day) has been dangerous fossil fuels. In the year 2000, they produced 337.12 exajoules; in 2016 they produced 466.83 exajoules, an increase of 129.71 exajoules, the equivalent of adding another United States (and then some) to the world energy equation, a United States running on 100% dangerous fossil fuels, the United States being the country with the absolute worst per capita energy consumption on the planet. Overall, world energy demand increased in that period by 155.96 exajoules, with 26.00 exajoules coming from additional wind, solar, bioenergy (including "traditional" biomass which is responsible for about half of the world's air pollution deaths), and hydro combined. Bioenergy provided more than half (13.60 exajoules) of this new so called "renewable energy."
By contrast, what I regard as the only truly safe and sustainable form of energy - owing to its high energy to mass ratio - nuclear energy, grew not at all, effectively, by 0.21 exajoules to a total of 28.51 exajoules.
Never underestimate the power of fear and ignorance to kill people, this to resounding applause.
This data may be found here: IEA 2017 World Energy Outlook, Table 2.2 page 79 (I have converted MTOE in the original table to the SI unit exajoules in this text.)
To return to the Nature news item, the soothsaying in the graphic above indicates that the predicted "expected" electricity demand will be 40,000 TWh in 2030 - thankfully a unit of energy - which translates into 144 exajoules.
Of course, if you ask most people, since most people know nothing at all about the subject of energy even as they think they do, they'll tell you that they believe that almost all of the increase will come from so called "renewable energy" by converting all of the world's pristine mountain tops into wind industrial parks, all of its deserts into industrial parks covered with glass, steel, aluminum and a plethora of exotic metals suitable for discussion in a toxicology course, and all of its continental shelves into industrial parks, with benthic zones covered with greasy whirlygigs stuffed with copper and lanthanides attached steel posts sunk into vast pillars of concrete.
Happily or unhappily, I cheerfully predict that this won't happen, since nearly 100% of the predictions make this claim and retrospective experience shows that nearly 100% of similar predictions have been 100% wrong.
I predict that current trends will continue and almost all of the world's electricity will come in 2030 from thermal sources, perhaps marginally more efficient that current thermal sources, gas, coal, oil and nuclear, all of which exhibit thermal efficiencies in the neighborhood of 33%. Even if, by the use of combined cycle thermal technologies, we managed to raise the thermal efficiency to something like 40%, 144 exajoules of electricity will imply a primary energy demand of 360 exajoules.
Since, because fear and ignorance have won the day, nuclear energy will not increase, thus implying a huge carbon cost to be dumped on every single living human being, plant and animal that will live in the future, impoverishing whatever it does not destroy.
Some text from the article:
These huge buildings are the treasuries of the new industrial kings: the information traders. The five biggest global companies by market capitalization this year are currently Apple, Amazon, Alphabet, Microsoft and Facebook, replacing titans such as Shell and ExxonMobil. Although information factories might not spew out black smoke or grind greasy cogs, they are not bereft of environmental impact. As demand for Internet and mobile-phone traffic skyrockets, the information industry could lead to an explosion in energy use (see ‘Energy forecast’)...
One of my favorite jokes about the failed, expensive and useless solar industry is that it is unable to run the servers dedicated to saying how great it is, but the real numbers suggest my joke is definitely an exaggeration, not that anyone in this day and age seems unwilling to engage in hyperbole, present company included:
Nobody today gives a rat's ass about the lives of children born today, by the way, they can go scratch, at least if we look at our policies and the undeniable fact that the rate of new carbon dioxide additions to the atmosphere is at the highest ever observed and it will be their problem, not ours to clean it up.
200 TWh is only about 0.72 exajoules. So called "renewable energy" including only solar, wind and geothermal was, as of 2016, 9.42 exajoules, or 1.6% of world energy demand to use the "percent talk" so called "renewable energy advocates so love, albeit after half a century of predictions about how it would dominate the world energy supply "by 2020."
It's clear from this figures that solar and wind could power all the world's data center servers (but not much else) as of now, so feel free to call me out on my joke in the future.
A little more text:
...Perhaps the most startling forecast of ICT’s future energy demand comes from Anders Andrae, who works on sustainable ICT at Huawei Technologies Sweden in Kista; he predicts that data-centre electricity use is likely to increase about 15-fold by 2030, to 8% of projected global demand1. Such dire numbers are controversial. “There have been many alarmist predictions of growing ICT energy use over the years, and all have proven to be bunk,” says Masanet. Last year’s IEA report estimated that although data-centre workloads will shoot up — tripling 2014 levels by 2020 — efficiency gains mean that their electricity demand might sneak up only by 3%2. ICT’s carbon footprint as a whole might even drift downwards by 2020, as smartphones take over from larger devices, researchers have suggested3...
I think it's possible the news item is open sourced, so feel free to read it yourself. It's a fun read, with all sorts of hopeful remarks about what so called "renewable energy" will do for the information industry, even if so called "renewable energy" has done nothing at all to arrest climate change, is doing nothing to arrest climate change, and will always remain incompetent to do anything about climate change, not that reality should ever interfere with popular faith.
Popular faith aside, we hit almost 412 ppm of carbon dioxide in the planetary atmosphere earlier this year and no one now living will ever again see a figure below 400 ppm. Right now, we are running about 22-23 ppm higher than we were running just ten years ago.
Have a nice day tomorrow.
The 1S-2P Lyman-α transition in antihydrogen has been observed.
Very, very, very cool...
The Lyman series played a huge role in the foundation of modern quantum mechanics when it was explained by the young Neils Bohr, bringing him to the forefront of physics, in the history of which, he played such a huge role.
Now a transition from this series has been observed in antimatter in an elegant experiment.
From the abstract:
From the introduction:
Because matter and antimatter annihilate each other when they meet, antihydrogen must be created and then trapped in strong, inhomogeneous magnetic fields in an ultrahigh-vacuum chamber. The ALPHA-2 apparatus (Fig. 1a) is designed to combine antiprotons from CERN’s Antiproton Decelerator15 with positrons from a positron accumulator16,17 to produce and to trap atoms of antihydrogen.
A picture of the apparatus:

The paper is apparently open sourced, and should you be interested, and inspired to read it yourself, it's here: Observation of the 1S–2P Lyman-? transition in antihydrogen.
Cool, I think, very cool...
Honoring the Mother of Modern Symmetry Mathematics, Emmy Noether.
A conference in London this week, the Noether Celebration, hopes to change that. It’s a welcome move. In a world where young scientists look for inspirational female role models, it is hard to think of a more deserving candidate.
Noether was born in 1882 in Erlangen, Germany. Her parents wanted all their children to get doctorates, so although many universities at the time did not formally accept women, she went. After graduation, sexist regulations prevented Noether from getting jobs in academia. Undaunted, for many years she lectured in Erlangen and, from 1915, at the University of Göttingen — often for free.
At the time, that city was the centre of the mathematical world, largely due to the presence of two of its titans — Felix Klein and David Hilbert. But even when Noether was being paid to teach at Göttingen and making her most important contributions, fate and further discrimination intervened: Hitler took power in 1933 and she was fired for being Jewish...
...The results that Noether published 100 years ago were, for her, a rare foray into physics, in which she was not particularly interested. Albert Einstein had just developed his general theory of relativity, and was struggling to understand how energy fitted into his equations. Hilbert and Klein were working on it, too, and asked Noether for help.
That she did help is an understatement. Noether’s expertise in symmetry led her to discover that the symmetries of a physical system are inextricably linked to physical quantities that are conserved, such as energy...
I came across this news item in the most recent issue of Nature:
Celebrate the mathematics of Emmy Noether
I plainly confess that I had no idea about Noether until encountering this article.
Apparently for a time she was not allowed to join formally the faculty at Gottingen and therefore lectured under Hilbert's name. She worked for no pay, just for the love of mathematics.
These are the world's most beautiful people, people who do great things only for the appreciation and love of beauty.

She reminds me of another great female contributor to science who also labored against prejudice against women in science and triumphed nonetheless, Lise Meitner, the true discoverer of nuclear fission. I wrote about Meithner elsewhere: The Deformed Nucleus, Neptunium and the Rain.
I thought it worth mentioning.
What do you get when you cross a mosquito with a rock climber?
Nothing. You can't cross a vector with a scalar.
Jean-Paul Sartre is sitting at a French cafe, revising his draft of Being and Nothingness.
He says to the waitress, "I'd like a cup of coffee, please, with no cream." The waitress replies, "I'm sorry, Monsieur, but we're out of cream. How about a cup of coffee with no milk?"
Can Biocoke Address the Anode CO2 Problem (Owing to Petroleum Coke) for Aluminum Production?
The paper from the primary scientific literature I'll discuss in this post is this one: Utilization of Biocoke as a Raw Material for Carbon Anode Production (Xianai Huang*, Duygu Kocaefe , and Yasar Kocaefe, Energy Fuels, 2018, 32 (8), pp 8537–8544)
The text of this paper has this interesting introduction:
The substantial dependence on carbon and electricity makes the electrolytic aluminum production process a major greenhouse gas (GHG) emitter. All of the carbon consumed in the electrolysis cell is eventually released to the atmosphere as mainly CO2 (plus some CO). In Canada alone, annual CO2 emission due to carbon anode consumption in smelters is about 1.2 × 10^6 tonnes.
In 2017, world production of aluminum metal represented 68.7 million tons, with the bulk of it being produced in China. This implies that the carbon release to the atmosphere from the electrodes alone, never mind the carbon released to provide electricity for aluminum production - which dwarfs the amount involved with the decomposition of electrodes - is on the order of 27.5 million tons per year.
The paper does not mention another product of the "burn up" of carbon anodes, which is CF4, carbon tetrafluoride, as the Hall process for aluminum releases copious amounts of CF4 (perfluoromethane), which according to the 5th climate assessment report has a global warming potential, a measure of how much worse it is than carbon dioxide, of 6,630, a factor of more than 230 times greater than methane itself.
According to the aluminum industry, the mean fluoride intensity of aluminum production is 0.58 kg "F"/ton Al. Chemically, all of this "F" is released as CF4. The mean atmospheric lifetime of CF4 in the planetary atmosphere, where the main sink is radiation, is on the order of 50,000 years.
Humanity has chosen - foolishly in my view - to bet the future of the planetary atmosphere on so called "renewable energy." Trillions of dollars have been invested in this pixilated adventure on solar and wind alone: UNEP Frankfurt Report, Investment in Renewable Energy, Table (Figure) 3, page 14
It did not work; it is not working; it won't work. The rate of increase of carbon dioxide concentrations is the highest ever observed by humanity. In the last ten years, this baneful bit of data has increased by more than 23 ppm. The reason that so called "renewable energy" did not work, is not working, and will not work - and was in fact abandoned in the early 19th century when the world's population was less than 1/7th of what it is today - is physics: Low energy to mass ratio systems result in mass intensity requirements that are not sustainable.
Despite the obvious failure of this strategy of betting the planetary atmosphere on so called "renewable energy" - this failure is a fact, an "inconvenient truth," if you will - enthusiasm for this nonsense remains unabated, although some serious questioning of it has been growing in volume in the primary scientific literature, chiefly around resource issues which raise the very real question of whether so called "renewable energy" is actually "renewable" or whether it is merely a scheme to trade one baleful resource and waste issue with another, all future generations of human beings and other living things be damned.
Among the questions raised is the question of aluminum.
A wonderful paper that has generated a considerable number of citations as well as lots of thought on my part and, I'm sure, many others, is this one: Metals for a low-carbon society (Olivier Vidal, Bruno Goffé & Nicholas Arndt, Nature Geoscience volume 6, pages 894–896 (2013)). While I am personally dismissive of a reference to the importance in a reduction of so called "radioactive waste," (the reduction of which I regard as a completely idiotic, if popular, idea, since we might realize, were we to come to our senses, that radioactive materials are essential to any hope we have of halting and cleaning up this on going environmental destruction) I do appreciate the realistic and often ignored comments with respect to so called "renewable energy" within this paper, specifically:
... If the contribution from wind turbines and solar energy to global energy production is to rise from the current 400 TWh (ref. 2) to 12,000 TWh in 2035 and 25,000 TWh in 2050, as projected by the World Wide Fund for Nature (WWF)7, about 3,200 million tonnes of steel, 310 million tonnes of aluminium and 40 million tonnes of copper will be required to build the latest generations of wind and solar facilities (Fig. 2). This corresponds to a 5 to 18% annual increase in the global production of these metals for the next 40 years. This rise in production will be added to the accelerating global demand for ferrous, base and minor metals, from both developing and developed countries, which inflates currently by about 5% per year5,6..
12,000 TWh is 43 exajoules, this on a planet where humanity was, as of, 2016, generating and consuming 576 exajoules of energy, 81% of which was generated using dangerous fossil fuels. (Even the wind industry's fantasies are useless.)
A graphic from that paper paints the picture starkly:

Below I will turn to the energy requirement of preparing coke itself, be it biocoke or dangerous fossil fuel based coke, but actually the biggest energy cost of the production of aluminum is not the preparation (or oxidation) of electrodes, it is the electricity itself.
The most energy efficient nation for the production of aluminum is China, which is also the world's largest producer. Chinese aluminum production requires 13,577 kwh/ton of electrical energy, as compared to 14,738 kwh/ton in North America, and a world wide average value of 14,151 kwh/ton, these figures coming from the 2017 data provided by the World Aluminum association.
From the global electric intensity figures and from the production figures in the link above showing that the world produced 68.7 million tons of aluminum in 2017, we can calculate that aluminum production required 3.50 exajoules of pure electricity. We may compare this with the electricity produced by the so called "renewable energy" industry including wind and solar (but excluding hydroelectricity) which as of 2016 was 9.42 exajoules.
IEA 2017 World Energy Outlook, Table 2.2 page 79 (I have converted MTOE in the original table to the SI unit exajoules in this text.)
Hydroelectricity produced 14.65 exajoules; the total energy production from so called "renewable energy" as represented by wind and solar and hydroelectricity combined being dwarfed by coal, which produced 157.21 exajoules in 2016. As I frequently point out, despite much popular delusional rhetoric to the contrary, the World Energy Outlook figures show that the fastest growing source of energy on this planet in the 21st century has thus far been coal, the growth of which was 60.46 exajoules. These figures obviate the fact that as of recent times, the actual energy consumption associated with the generation of electricity - and this is particularly true in China - is thermal (generally steam) energy which has a thermodynamic efficiency in general on the order of 30-35% suggesting that the total energy demand for aluminum production in 2016 was on the order of 10 exajoules, not 3.5 exajoules.
Now let's consider what might be involved in raising world aluminum production to the 310 million metric tons that the Nature Geoscience paper suggests might be required to raise world wind energy production to a larger fraction of the world energy supply, while still not eliminating dangerous fossil fuel requirements. If we use the rough Chinese efficiency figures of 13,600 kwh/ton this suggests that the annual world energy demand just for aluminum, never mind the other energy intensive materials going into the production of wind turbines would be on the order 15 exajoules, a figure that exceeds all the energy production by all the world's solar and wind production in 2016 - 50 years into the unrestrained cheering for this cockamamie and failed gamble on which the planetary atmosphere was foolishly bet. It almost presents a Rube Goldberg type perpetual motion machine, particularly since the Danish data I referenced in this space some time ago gives a mean lifetime for wind turbines that is less than 20 years:
Average Lifetime of Danish Wind Turbines, as of February 2018.
The carbon released by the degradation of electrodes each year, to produce 310 million tons of aluminum per year would rise from under 28 million tons to 124 million tons, with a small but highly disturbing fraction being released in the form of the intractable greenhouse gas CF4 described above.
Note that I have not referred to the steel mentioned in the Nature Geoscience commentary. The coke intensity of steel dwarfs that of aluminum. According to the World Steel Association, current steel production requires about 1.1 billion tons of metallurgical coal to produce - processed again into coke- and 2.1 billion tons of iron ore to generate 1.7 billion tons of steel each year.
The energy intensity of steel - an artifact of the requirement to intensely heat coal to make coke - is reportedly, at the World Steel Association’s link, 20 gigajoules per ton. This implies that to make enough wind turbines to produce 43 exajoules per year while grinding birds into feathers and puree and bats into bat guts and slivers of wing skin, the annual production of steel (3,200 million tons) would require 64 exajoules of new energy per year, just for steel.
There was thus, perhaps, a prescient reason that Cervantes chose windmills for Don Quixote to attack as an exercise in absurdity.
The preparation of “coke” from either coal, petroleum refinery residuals, and/or biomass as we will discuss shortly in when we turn to the paper referenced in the opening paragraph of this post, requires energy, energy of a particular quality, sensible heat, which despite some tiresome daydreaming on the topic to the contrary, is generally not available from so called “renewable energy,” at least without the energy intensive and wasteful utilization of resistive heating derived from electricity. This is especially true of intermittent energy which is randomly available owing to the “zeroth law of thermodynamics” which is merely a statement of the readily observed fact that in the absence of the addition of energy, two systems in contact with one another will come to thermal equilibrium at which their temperatures are equal. Although the description of purely adiabatic systems is essential to the teaching and consideration of thermodynamics, as a practical matter, in non-ideal reality, they don’t exist.
Of course, one is further compelled to imagine the case where a plant manager calls his staff out of bed during the night at two in the morning to announce “Come to work to make bio-coke! The wind is blowing!” (This sarcasm ignores the use of biomass, which for centuries was used to make and refine iron and steel. However, besides being responsible for half of the seven million air pollution deaths each year, biomass combustion was related to the historic deforestation of Europe in the 16th and 17th centuries, which was part of the impetus driving the European desire to rape North and South America, the desire to have wood. This fact should stimulate some thinking, since huge portions of European forests were destroyed on a continental scale simply for wood construction and combustion when the population there was merely a tiny fraction of what the current world population is
The unsustainable combustion of biomass was responsible for the production of 56.69 exajoules of energy in 2016, up from 42.83 exajoules in the year 2000, although the majority of this energy was used for domestic purposes – thus killing huge numbers of them - by the world’s most impoverished people, about whom we couldn’t care less, the use of biomass notwithstanding the destruction of the Mississippi River delta ecosystem because of nutrient run off from Iowa’s corn ethanol production for “renewable” biofuel for our ever precious cars.
This now brings us to a discussion of the paper evoked at the outset of this post.
The authors continue with the introduction excerpted above thus:
The truth of this statement about whether or not biocarbon materials can be considered as "GHG neutral," greenhouse gas neutral, is actually dependent on the source of the heat to make the biocarbons, because the heat is considerable.
From the materials section:
2.2. Calcination of Biocoke. Table 1 shows the calcination conditions for all of the materials used in the study. In the first part of In the second part of the study, one biocoke (BCO-1) was chosen for anode production. This required large amounts of biocoke addition to anodes, and biocoke for this purpose was produced using a lab furnace (F in Table 1) using the same calcination conditions as the ones in the TGA. The large sample was placed in a container that was surrounded by filler coke (calcined petroleum coke used for protection).
Table 1 lists 7 samples, one of which is commercial biochar from an industrial partner; another of which is commercial petroleum coke. The condition for the formation of the former is assumed to involve heating at 450C; the condition for the latter is assumed to be 1200C. The latter may also be assumed to have used heat generated by the combustion of dangerous fossil fuels.
All of the other 5 samples were prepared under a stream of nitrogen gas (to prevent combustion) at different heating rates using a precise device called a thermogravimetric analyzer, which is essentially a sensitive analytical balance that measures mass loss as a function of temperature; the heater is internal to the device and the rate of increase in temperature can be carefully controlled. The heating rate for the preparation of 5 samples ranged from 0.2C/min to 40C/min. All the samples were heated to 1200C. Thermogravimetric devices are capable of only handling small samples. One of the samples, the one heated at the slowest rate, 0.2C/min, was duplicated in preparation in a laboratory furnace, this to allow the actual preparation of electrodes. (In some of the graphics below, this material is designated “F”.)
The samples were then characterized using various analytical techniques to determine surface area, morphology, etc, an important parameter being the density of the samples. Here is a graphic showing the density findings for the biocoke samples:

The caption:
One of the things I'm learning as I try to keep up with my son's education in Materials Science Engineering is the extreme importance of morphology on the properties. In my chemical career, I've been more focused on molecular structure than on morphology, and this is not a good idea, particularly for species intended to perform liquid or gas/solid interfaces, for examples catalysts as well as in this case, the formation of electrodes. (In my defense, the chemistry in which I’ve been involved generally involved the formation and breaking of bonds; consideration of micro morphology has seldom been required of me.)
The authors provide us with SEM images of their biocokes:


The caption:
The description production of the electrodes is where the authors disabuse us of any super optimistic feeling we may have had that these electrodes are even close to being fossil fuel free. They write:
Actually, to characterize these anodes as a biobased is somewhat misleading; the percentages of biologically sourced carbon in them is rather small:
The authors discuss the chemical differences between "biocoke" and "petroleum coke" by discussing the FTIR spectra which is here:

The caption:
The authors discuss the main differences in the spectra of these two in terms of the heteroatom species present, in particular oxygen species. This difference is unsurprising. One of the main problems with pyrolytic "bio-oils" which can be distilled from biomass by heating it in the absence of oxygen is the lack of stability, almost all of which can be attributed to oxygenated species therein. A great deal of research has been done to address this problem. (From my perspective, this limitation is best addressed by total reformation of the biomass in the presence of high temperature steam to give "syn gas," using nuclear heat for this purpose.)
Anyway...
A spectral technique related to IR (inasmuch as it pretty much involves the same wavelengths) is Raman spectroscopy, although in Raman spectroscopy, shifts are measured and in FTIR, main frequency is measured.)
Here is the Raman spectra:

The caption:
I've not had occasion in my career to use Raman spectroscopy, although I've problem contemplated thousands of FTIRs, although it turns up fairly frequently in the things I read and sales people call me up or send me emails from time to time offering Raman devices (including hand held Raman devices). The text from the paper gives a nice flavor for the technique and can inspire one to some deeper reading:
We then have a picture of anodes formed containing differing quantities of biocoke:

The caption:
While I'm certainly not competent to appreciate how the appearance might affect the performance of the electrodes, it appears that one factor involved is porosity.
The electrical resistivity is measured and graphically represented:

The caption:
These effects are discussed in the text:
The intersections of the highly resistive regions in two directions show the position of defective regions in the anode. For the anode produced with the addition of finer biocoke particles (Figure 6e, particle size less than 45 ?m), the resistivities are lower in comparison to the other anodes made with larger biocoke particles. This anode also displays a resistivity distribution similar to that of the standard anode. The finer particles have lower porosity and thus decrease the porosity and consequently the electrical resistivity. Also, the resistivity distribution in both directions is more uniform.
Other graphics in the paper refer to the density of the electrodes, reactivity with air and flexural strength, all important factors.
Probably the most important from the perspective of climate change as represented by the both the efficiency of the process as well as its carbon footprint is "dusting" which is a reflection of activity related to a chemical reaction which, in my view, would probably be key to any hope future generations have of cleaning up the mess we have so cynically dumped on them with our indifference and contempt. This is the Boudouard reaction, which is shown in two forms under the line in the graphic below:
(This reaction is responsible for the formation of soot under certain conditions during the combustion of the dangerous fossil fuel natural gas, which largely consists of methane which lacks a carbon carbon bond and thus would be expected to burn "clean," wherein someone accepts the dubious claim that carbon dioxide is a "clean" combustion product: It isn't.)
It would be nice if the oxidized species in the aluminum smelting process was the oxide ion which would be oxidized to oxygen gas. But in aluminum smelting - the process for the electrochemically driven smelting of aluminum is known as the "Hall Process" the oxidized species is carbon. (At the conclusion of this post, I'll briefly refer to another electrochemical metal reducing system in which under ideal conditions the oxidant can be oxygen gas.) Carbon can generally be oxidized to one of two forms, carbon dioxide or carbon monoxide. (Other oxides of carbon are known, notably certain graphene oxides as well as suboxides, but they are not known to be a factor in the Hall process.)
There is, open sourced on the internet, an excellent overview of the Hall process. It is, for anyone who might actually be interested, here: The Aluminum Smelting Process.
The link showing the chemical reactions (as well as the thermodynamic values) involved in the reduction of alumina to the metal is given in the link therein, this one: Process thermodynamic - Enthalpy
The practical reaction is described in equation 4 on this page:

The most efficient (but not generally observed practically) process in this reaction would be the case wherein x = 1, in which case the reaction reduces to equation 1 on this page:

To the extent that x < 1, the reaction requires larger amounts of carbon to take place and thus is less carbon efficient. This is obviously not a good thing.
The mechanism by which carbon monoxide is formed is very much involved with the equilibrium values of the Boudouard reaction:
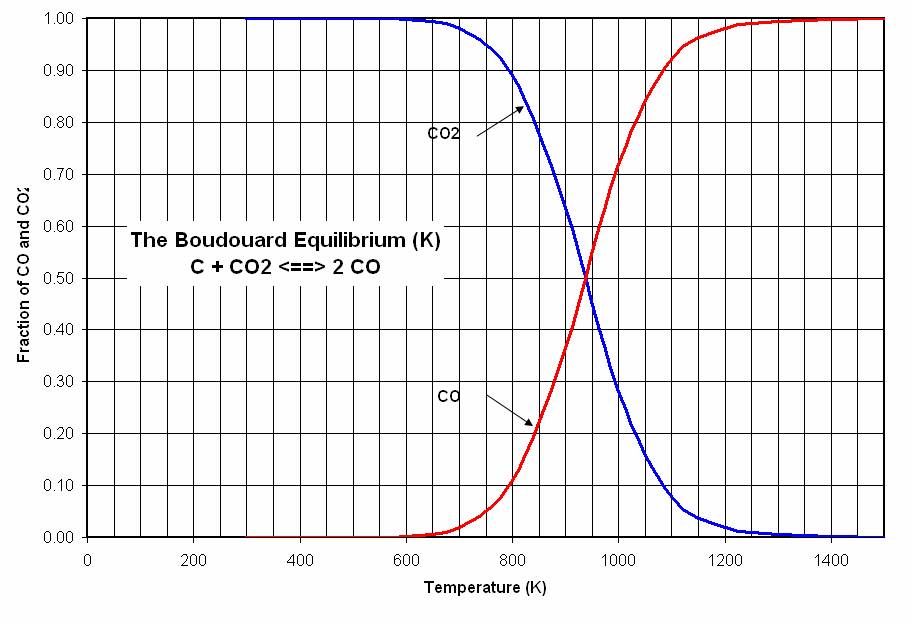
However, it is not the case that carbon formed by the disproportionation of carbon monoxide into elemental carbon and carbon dioxide will deposit on the electrode. It may form in the reaction mixture as a fine carbon dust. This is "dusting." Dusting still raises the amount of carbon required, although in this case, the "dust" is not released into the atmosphere, although it can and does have negative effects on the process.
The dusting for the electrodes discussed in this paper are described in two graphics:

The caption:

The caption:
The authors promise to do more research, in order to raise the the content of biocoke beyond 5%.
It is worth noting that reactions related to the Boudouard reaction and often discussed in the scientific literature allow for the facile production of carbon monoxide by using carbon dioxide and an oxidizing agent for biomass instead of oxygen, with sensible heat being used to drive the reaction. In the case where the sensible heat were to be nuclear energy, this series of reactions would be carbon negative, in effect, removing carbon dioxide from the atmosphere.
Also the thermochemical reduction of carbon dioxide by splitting it, also requiring heat, is known. Personal favorites are the zinc carbon dioxide splitting cycle, Ind. Eng. Chem. Res., 2013, 52 (5), pp 1859–1869 and cerium based cycles, one of which is described here: Energy Fuels, 2015, 29 (12), pp 8168–8177. Regrettably, to obtain funding, the heat source for these reactions is described as being solar thermal, although every ersatz solar thermal plant attempted as proved to end up as defacto gas plants with extremely low reliability, large operating costs, and extremely questionable environmental impacts. They have not worked; are not working and will not work. Happily though this same chemistry can be applied to nuclear systems which are more reliable, and less environmentally odious.
I would note that it is conceivable - although far from practicality in a demonstrated sense, to use Boudouard carbon to make electrodes, probably with a lower carbon impact than the biocoke discussed herein. That at least is what I speculate.
A new electrochemical process has been under development in recent years, and is most widely being explored to manufacture titanium, a common element that can be reduced to a strong heat resistant metal that is lighter than steel. The expense, the high cost, of titanium metal is been tied the cost of reducing it, which is the metallothermic Kroll process which is awkward, difficult and highly energy intensive. (Historically aluminum was made in a similar fashion, and until the discovery of the Hall process, it also was extremely expensive, so expensive that Napoleon III had dinnerware made from it in order to impress his guests with his wealth.)
The new process is the "FFC Process," named for the academics who discovered it, Farthing, Fray and Chen. This process takes place in molten calcium choride as opposed to the sodium aluminohexafluorate (cryolite) utilized in the Hall process.
Here is a paper discussing some aspects of the FCC process by one of the discoverers of the process, Derek Fray: Alexander, Schwandt and Fray, Acta Materialia 54 (2006) 2933–2944
In many incarnations of the FFC process, the anode is carbon, with the same oxide products as in the Hall process, with CF4 being replaced by easier to remove (and destroy) chloromethanes, up to an including carbon tetrachloride. However in recent years ceramic anodes have been explored, including some by Fray himself: Materials Research Bulletin 44, 8, 5 2009, 1738-1742)
In the case of electrodes like these, under appropriately controlled conditions, the oxidation product can be oxygen gas. This is a good thing.
Derek Fray, by the way, is a man after my own heart, since one of his activities has been the electrochemical processing of used nuclear fuel, which in my view, is almost certainly the best process - perhaps in concert with ion selective membranes, either solid or liquid, for the recovery of the multiple valuable materials found in used nuclear fuel.
His web page is here: Professor Derek Fray, FRS
If we are at all serious about addressing climate change - and there's no evidence that we actually are - nuclear energy is the only sustainable means to doing it.
To the extent that electrochemical reduction of ores to metals can be made sustainable - we're not there yet by any means - carbon free energy (only nuclear energy practically meets this standard) and carbon free anodes are almost certainly required. I do believe that certain classes of modern metalloceramics - my son has been studying these - may help us along this road to the "sustainable anode."
I'm not sure that this long and highly esoteric post is of much interest here - although it reflects a certain reality with respect to the question of so called "renewable energy" is really "renewable" - but I rather enjoyed writing it, since I learned a lot of things in the process that give me hope that all is not actually lost.
I trust you will have a pleasant Sunday.
Of the three McCain speeches I watched this morning, the most moving for me was Biden's.
Megan McCain's speech was, of course, the most personal; Obama's the best broad look at what it means to be an American, but Biden's, touched me most deeply on the meaning and depth of grief, which of course, Joe Biden unfortunately knows too intimately.
His personal respect for McCain - derived from personal knowledge of the man - made me think anew about the man, but the compassionate and deep and personal way he addressed McCain's family moved me deeply.
Joe Biden is a great man by virtue of being a great human being.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,509