NNadir
NNadir's JournalAnyone who requires that they can only learn facts from people who are nice to them is a fool.
I have learned quite a bit from people I considered obnoxious, but I'm not quite as self-absorbed as some others. Facts are facts, and how one states them will not make them less true.
I hear this nonsense a lot, because I'm not inclined to be saccharine in inappropriate settings. I use this analogy every time I hear it. A person sees a man standing on a track with a train rushing at him and yells, "Hey you fucking asshole, get off the track! A train's coming!" and the guy on the track says, "Ask me nicely and I'll do it."
Now in the analogy, the fool is killing himself. In the broader case, people cheering for so called "renewable energy" are killing more than themselves.
The solar/wind/gas/coal/petroleum scam is killing the planet. We spent trillions of dollars on solar and wind and the rate of accumulation of dangerous fossil fuel accumulations are rising, not falling. In 2000 that rate was roughly 1.5 ppm/year. Now it's 2.45 ppm/year. For this entire century, anti-nukes advocates of so called "renewable energy" have been crowing over their "success."
I'm not inclined to indulge people cheering for this outcome with the same tiresome rhetoric and be all sweet and indulgent. I don't cheer for delusional people at all. I don't indulge Trumpers; I don't indulge anti-vaxxers and I don't indulge anti-nukes. To my way of thinking they are all equivalent.
Why am I so angry? Oh I don't know...
Maybe I give a shit that scientific journals are reporting, as I noted yesterday, Extreme temperatures in major Latin American cities could be linked to nearly 1 million deaths. The original source for that post was the prominent Journal of the AAAS, Science. I also linked the original paper in Nature Medicine. Nature published another news item recently as well: Extreme heat already claims lives in Latin American cities — and the toll is set to rise. This is going on while whiny brats carry on about Three Mile Island.
Excuse me if these unnecessary deaths don't make me all giggly and cute when I hear recycled bullshit about hydrogen buses and other bourgeois chanted bullshit.
"Renewable energy" heaven, Germany, is burning coal continuously; they don't stop; they can't get enough of that poison to burn. Oh, and they shut their nuclear plants in a paean to fear and ignorance.
I'm not a child; I'm a grown up, not someone watching cartoons and confusing them with reality.
I know what I see when I see the picture in the OP. I see a huge stretch of land squandered to produce trivial amounts of energy, as much as could be produced in a few liters of nuclear fuel. I see this energy being produced on a vast stretch of land irregularly and unreliably, with the requirement for redundant dangerous fossil fuel systems. I see a pile of semiconductors that will be electronic waste in 20 years. I see chemical reactors 5 stories high filled with silicon halides by the thousands. I see children digging cobalt in central Africa, quite possibly under extreme temperatures, to make batteries to try to put a band aid - one that won't work - on this unreliable junk. I see people dying from extreme heat when the power fails. I see toxic leachates spread all over the world because people can't think, can't understand the relationship between energy density and sustainability.
Other people, see anti-nuke heaven. I'm not going to pretend I have use for these people. I don't.
Nuclear energy saves lives; it follows that anti-nukes kill people.
The data supporting these statements can be found here: Prevented Mortality and Greenhouse Gas Emissions from Historical and Projected Nuclear Power (Pushker A. Kharecha* and James E. Hansen Environ. Sci. Technol., 2013, 47 (9), pp 4889–4895)
This highly cited and highly read paper is open sourced. Anyone can read it. The data therein is irrefutable in my view.
Now if someone wants to go off and sing Kumbaya while the fucking planet burns - and it is burning - and carry on about hydrogen filling stations for their stupid automobiles, I'm not going to be able to stop them from doing it. My experience is that people can't be talked out their fondness for ignorance and wishful thinking. If they could be, we wouldn't be looking at concentrations of the dangerous fossil fuel waste carbon dioxide of over 420 ppm, less than ten years after we first saw concentrations over 400 ppm. But we are seeing readings over 420 ppm. At this rate, we'll be looking at 450 ppm in 12 years.
I'll probably be dead by that time, but regrettably, I will bear in history the shame of my generation of benighted, distracted, bourgeois assholes.
Have a lovely, wonderful evening.
Extreme temperatures in major Latin American cities could be linked to nearly 1 million deaths
I came across this as news item in Science: Extreme temperatures in major Latin American cities could be linked to nearly 1 million deaths.
Subtitle:
Science 28 JUN 2022 BY RODRIGO PÉREZ ORTEGA
An excerpt:
With climate change, heat waves and cold fronts are worsening and taking lives worldwide: about 5 million in the past 20 years, according to at least one study. In a new study published today in Nature Medicine, an international team of researchers estimates that almost 900,000 deaths in the years between 2002 and 2015 could be attributable to extreme temperatures alone in major Latin American cities. This is the most detailed estimate in Latin America, and the first ever for some cities.
Most studies that link extreme temperatures with mortality in cities have been done in North America, Europe, and China. “There’s relatively little locally generated knowledge that’s specific to the Global South,” says Ana Diez Roux, an epidemiologist at Drexel University who co-authored the new study. “Latin America, in particular, is a region that has not received a lot of attention.”
And the new paper has a much better representation of urban areas in Latin America than previous studies in the region, says Antonio Gasparrini, an environmental epidemiologist at the London School of Hygiene & Tropical Medicine. “So, this is already an improvement.”
To estimate how many people died from intense heat or cold, researchers with the Urban Health in Latin America project—which studies how urban environments and policies impact the health of city residents in Latin America—looked at mortality data between 2002 and 2015 from registries of 326 cities with more than 100,000 residents, in nine countries throughout Latin America. They calculated the average daily temperatures and estimated the temperature range for each city from a public data set of atmospheric conditions. If a death occurred either on the 18 hottest or the 18 coldest days that each city experienced in a typical year, they linked it to extreme temperatures. Using a statistical model, the researchers compared the risk of dying on very hot and cold days, and this risk with the risk of dying on temperate days. They found that in Latin American metropolises, nearly 6%—almost 1 million—of all deaths between those years happened on days of extreme heat and cold...
The full original paper, in Nature Medicine is here: Kephart, J.L., Sánchez, B.N., Moore, J. et al. City-level impact of extreme temperatures and mortality in Latin America. Nat Med (2022).
I believe the full paper is open sourced.
...but...but...but...but...Three Mile Island!!!!!!!
The ORNL Inventory of Isotopically Pure Actinide Targets for Super Heavy Element Synthesis.
I stumbled across this one while wandering around in the literature:
J.B. Roberto, C.W. Alexander, R.A. Boll, J.D. Burns, J.G. Ezold, L.K. Felker, S.L. Hogle, K.P. Rykaczewski, Actinide targets for the synthesis of super-heavy elements, Nuclear Physics A, Volume 944, 2015, Pages 99-116
These are the targets utilized to synthesize the super heavy elements, one of which is the halogen Tennessine, element 117. The inventory of targets is at ORNL, a very cool place if you've ever been there.
The inventory of the targets:

There's some cool descriptions of how to make these elements in the paper.
Interesting I think.
Concentrations of trifluoroacetic acid in water have increased 6 fold since 1998 in California.
I will only briefly note this paper I've just come across: Increases in Trifluoroacetate Concentrations in Surface Waters over Two Decades Thomas M. Cahill, Environmental Science & Technology 2022 56 (13), 9428-9434.
I have been very interested in the mechanism of radiolytic degradation of PFAS, a rising toxicological concern coming under the rubric of "forever chemicals." TFA is a potential degradation product of radiolytic degradation, if not carried to completion. TFA is also a potential product of the slow bioremediation and thermal remediation of these compounds.
However in this case, this is not the likely source according to the authors. They suggest it arises from the degradation of some of the HFCs that replaced CFC's because of the Montreal Protocol, the only successful international environmental treaty ever having broad international agreement.
Some text from the introduction of the paper:
Attention shifted away from TFA to the longer fluorinated chain acids in the late 1990s and early 2000s when the longer chain perfluorinated compounds, such as perfluorooctanesulfonic acid (PFOS), were discovered to be ubiquitous in the environment. Unlike TFA, these longer perfluorinated acids had both high animal toxicity and the potential to bioaccumulate, so they represented a more immediate risk to both humans and the environment. Research into TFA dwindled with only a few recent ambient measurements being reported, (15,16) while most of the remaining research focused on suspected point sources such as fluorochemical production plants (4?6) and fire-fighting training sites. (10)
However, the use of first-generation fluorocarbon refrigerants, such as HFC-134a, continued to rise and the atmospheric concentrations climbed along with them. Between 1998 and 2021, the atmospheric concentrations of HFC-134a increased approximately14-fold from 8.7 to 121 ppt at the Mona Loa Observatory, Hawaii, (17) so the amount of TFA formed in the atmosphere would be expected to increase by a similar value. Furthermore, HFC-134a is being phased out and its apparent replacement, namely hydrofluoroolefin-1234yf (HFO-1234yf), has a higher product yield for TFA (near 100%) (18) compared to HFC-134a (approximately 30% depending on pressure and temperature (19)), which would also suggest that more TFA will be forming in the atmosphere.
Two figures from the text, and a table of results:

The caption:

The caption:
A table of results:

Lovely.
The carbon fluorine bond is one of the strongest known and breaking it generally requires energy at least in the UV range.
The "Improved" Siemans Process for Producing Polysilicon for Solar Cells.
I keep hearing over and over and over, year after year, decade after decade, that the world will be saved from climate change by solar cells and batteries. None of this endless talk has prevented the atmospheric concentrations of the dangerous fossil fuel waste carbon dioxide from being over 420 ppm for the last 14 weeks of this year, 2022, less than 10 years after it first went over 400 ppm in 2013.
Of course, one cannot read any scientific journal without hearing about improving batteries, recycling them, and how wonderful the solar nirvana will be some day, "some day" having not come, is not here, and I think, won't come.
The environmental implications of this scheme have escaped much attention in the popular press, but increasingly one sees more and more questions about this topic in the scientific press.
I don't really follow the chemistry of solar cells all that much, since I find the issue to be of low value but occasionally I'll take a peek.
Tonight I came across this paper: A SiCl4-Assisted Roasting Approach for Recovering Spent LiCoO2 Cathode
Mengting Li, Beilei Zhang, Xin Qu, Muya Cai, Dongxu Liu, Fengyin Zhou, Hongwei Xie, Shuaibo Gao, and Huayi Yin
ACS Sustainable Chemistry & Engineering 2022 10 (26), 8305-8313.
It contains this text about the production of polysilicon, the most popular component of solar cells, citing an "improved" process:
Ten to fifteen tons of a highly toxic compound per ton of polysilicon, an "improvement..."
You don't say?
I imagine a few Bhopal type events in there with a leak, which were solar energy to become a significant form of energy - I think it won't - would increase in probability. This won't excite many scare stories over at the Union of Concerned "Scientists" of the ilk that the moron Ed Lyman produces daily about nuclear energy, without a single whimper about the hundreds of millions of deaths from air pollution that took place during his career in insipid whining, but I suspect over all, because of the extremely low energy to mass ratio of solar energy, it would become inevitable were the industry to matter, which it doesn't.
In order for the SiCl4 treatment of LiCoO2 cathodes to treat all of the SiCl4 waste generated, the masses of these products would need to be perfectly balanced, but that won't happen either.
Have a nice day tomorrow.
Gender Fluid Person Named to Manage Used Nuclear Fuel.
From Sam Brinton's Linkedin Page:
But goodness is this a time for celebration! It’s really really official! The beautiful irony that the months-long process of getting me into this role culminated in a Pride month start date is not lost on me. As one of if not the very first openly genderfluid individuals in federal government leadership, I was welcomed with open arms into the Department of Energy all the way up to the Secretary whom I shared the stage with in a Pride month celebration panel just today. To clarify, I am not a Biden appointee (despite what was reported) and instead serve as a career employee in the Senior Executive Service - I intend to be serving my country in this role through many many presidencies.
I’ve prepared for this moment in a technical sense for a decade. Graduating with not one but two degrees from MIT led to working at multiple think tanks where I produced the first-of-a-kind reports and maps on consent-based siting and advanced reactor innovation. Being the first employee of one of the world’s first nuclear waste start-up companies led me to innovate and drive the national conversation of nuclear waste management into the future. And now, I lead a staff of hundreds and a budget of millions (with a Nuclear Waste Fund I’m responsible for at over $45 billion) as the leader of the office overseeing the management of the nation’s spent nuclear fuel.
Thank you for being the community who believed in me. You got me through some dark days these past few months and I’m eternally grateful. Now it's time for me to make my mark as the Deputy Assistant Secretary...
Sam Brinton

Q&A with Nuclear Engineer, Presidential Adviser & Conversion Therapy Activist Sam Brinton
I may be an old fat bald straight white guy, but I particularly relate to this remark they made in the above interview:
Brinton: THIS is my best argument for my decision to become a nuclear engineer. Think about this: A nuclear engineer is constantly fighting the misperception that they are Homer Simpson and killing the environment with green glowing goo or about to build a nuclear bomb. They are constantly correcting public perception. And that’s what an LGBTQ person is doing too. We are helping our parents realize their idea of who we would marry isn’t necessarily the same. We are showing the world we are more than the stereotypical identities they see on TV. That’s why I think us LGBTQ nuclear engineers are the best nuclear engineers.
Ukrainian mathematician becomes second woman to win prestigious Fields Medal
From Nature News: Ukrainian mathematician becomes second woman to win prestigious Fields Medal
Subtitle:
Davide Castelvecchi, Nature July 5, 2022
Excerpt:
“All of the medalists are incredibly deserving and talented, showcasing the vibrancy of mathematical research across the globe,” says Bryna Kra, a mathematician at Northwestern University in Evanston, Illinois, who is president-elect of the American Mathematical Society.
Viazovska, who is based at the Swiss Federal Institute of Technology in Lausanne (EPFL), is the second woman ever to earn the award. She is best known for her solution of the sphere packing problem — finding the arrangement of spheres that can take up the largest portion of a volume — in eight dimensions.
In a three-dimensional space, the most efficient way to pack spheres is the pyramid arrangement, akin to how oranges are packed on trays in a grocer’s shop (proving this mathematically was extremely hard and was the subject of a tour-de-force paper in the 1990s). But in four or more dimensions, very little is known, says Henry Cohn, a mathematician at the Massachusetts Institute of Technology in Cambridge. “It’s this horrific gap in our knowledge — almost embarrassing,” said Cohn in an address following the Fields Medal announcement. Viazovska introduced new techniques into the problem that came from number theory and the theory of symmetries in eight dimensions. “Given how poor our understanding is in other dimensions, it’s really miraculous that Maryna was able to get this exactly,” Cohn added. More recently, Cohn worked with Viazovska and others to extend the result to 24-dimensional space.
“Viazovska invents fresh and unexpected tools that allow her to jump over natural barriers that have held us back for years,” says Peter Sarnak, a number theorist at Princeton University in New Jersey...
... The Fields Medals and other IMU prizes are normally announced at the opening of the International Congress of Mathematicians (ICM), which takes place every four years. This year’s congress was scheduled to begin on 6 July in St. Petersburg, Russia, but the plan was scrapped following Russia’s invasion of Ukraine in February. Instead, the awards ceremony was moved to Helsinki and the congress will take place as a virtual event.
“We condemn the madness, the injustice, and the irreversibility of war that threatens the very existence of humanity,” wrote four members of what had been the local organizing committee statement on 27 February.
The committee that chooses the Fields winner — whose members’ identities were kept secret until today — reportedly made its decision before the invasion...
Another winner of the Fields medal, June Huh, is also an interesting person. Now a Professor of Mathematics at Princeton, he was a high school drop out in Korea and was rejected at many Universities when he applied to Ph.D. programs because of his poor undergraduate grades, including many failed courses.
The Rogue Supreme Court has declared the Constitution unconstitutional.
It is now asserting itself as the inquisition courtesy of Mitch McConnell and his enablers, including Mitt Romney and Susan Collins, both of whom pretend to be "conservative" and both of whom have worked to destroy our Constitutional government.
They must be stopped.
Uncovering the Key Features of Dysprosium Flows and Stocks in China
I have just come across this paper: Uncovering the Key Features of Dysprosium Flows and Stocks in China
Shijiang Xiao, Yong Geng, Hengyu Pan, Ziyan Gao, and Tianli Yao Environmental Science & Technology 2022 56 (12), 8682-8690.
There are 14 lanthanide elements, with yttrium making 15 although it is not strictly an "f element" although its chemistry is very similar. Most lanthanide (aka "rare earths" ) deposits are dominated by just two, cerium and yttrium, with the other, with the lighter 6 including cerium as well as lanthanum, praseodymium, neodymium, samarium, and gadolinium dominating the remainder. (Europium can behave differently than most lanthanides and is sometimes depleted in ores. Promethium does not occur naturally; it is radioactive, with a short half life, but can be obtained, in very small quantities - because of its high neutron capture cross section - from used nuclear fuels.)
Dysprosium is a "heavy" lanthanide, and as such is relatively rare. Nevertheless it is in high demand, hence this article.
The largest application, as one can see from the open abstract, is to build wind turbines, which after decades of cheering and the destruction of huge tracts of wilderness to make industrial parks, remains a trivial form of energy, even though the fate of the planet's atmosphere has foolishly been bet on them.
From the opening text of the full article:
China has the largest REE reserve with an amount of approximate 44 megatons (Mt, 1 Mt = 1,000,000 tons), accounting for 37% of the global reserve in 2019. (14) As for Dy, it is estimated that the global dysprosium oxide (Dy2O3) reserve is 1.62 Mt, referring to 1.41 Mt Dy metallic equivalent. (14) China has the largest Dy reserve with an overall amount of 1.23 Mt, accounting for 87% of the global reserve. (14) Such reserve exists for several common rare earth minerals, such as monazite, xenotime, bastnasite, and ion-adsorbed clays (IACs). In particular, the Dy-rich IACs are distributed only in Southern Chinese provinces, such as Jiangxi, Fujian, Guangdong, and Hunan. However, the accurate flows and stocks of China’s Dy cycle remain unclear.
Material flow analysis (MFA) is one widely recognized method to characterize material flows through the anthroposphere. (15) MFA is capable to track material flows through a specified system boundary and identify how one material transforms and accumulates over its lifecycle based on the principle of mass balance. (16) This method has been employed to analyze most mineral elements and several REEs within different regions and periods, such as aluminum, tungsten, graphite, and neodymium. (17?20) As for the Dy cycle, the existing MFA studies mainly focus on the Dy flows associated with NdFeB in Japan, (1) the impacts of the increasing demand for neodymium (Nd) and Dy on the supply and demand of the host metals and other companion REE in wind power in the US, (12) Dy stocks and flows in NdFeB magnets among 18 various products and its recycling potential by 2035 in Denmark, (21) and the effectiveness of reducing Dy demand from low-Dy NdFeB magnets and promoting NdFeB magnets recycling in Japan for 2010–2030. (22)
The term used in this opening statement, "clean energy" is one I personally find objectionable. There's nothing "clean" about wind and electric cars.
I recently attended an online lecture put on by the Irving Institute at Dartmouth by a scientist whose name escapes me about the "recycling" handwaving that goes on, by the way. She pointed out that in use materials are not available for recycling, and where use is growing rapidly the demand must be met by mining.
Some graphics from the paper:

The caption:
Some frame work on the flows:
(1) Domestic flows: domestic flows are the basic Dy flows, covering mining, fabrication, manufacturing, and use stages. They are calculated based on the amounts of primary products, intermediate products, final products, and Dy contents in different products. Various Dy forms are transformed into unique metallic Dy flows. These final products and their Dy contents are listed in Tables S1 and S2.
(2) Trade flows: trade flows reflect the Dy import and export amounts at five stages with various forms. These Dy-containing products are coded by the Harmonized Commodity Description and Coding System of China and listed in Table S3. The trade flows are equal to the traded product amounts multiplied by their corresponding Dy contents.
(3) Loss flows: in this study, the Dy losses are assumed to occur in the R&S stage and fabrication stage, with figures of 10 and 30% respectively. (1,26) These loss flows are calculated by multiplying the production amounts and their loss rates.
(4) Supply-demand flows: Dy flows are calculated by the top-down approach in the R&S stage, while the bottom-up approach is used to estimate the demand for Dy in the fabrication and use stages. Hence, there is a mismatch between the Dy-containing concentrate supply and compound demand, and this gap is considered to be composed of hibernating stock and illegal mining.
(5) In-use stocks: a bottom-up approach is applied to estimate the in-use Dy stocks through the accumulation of net flows since the base year of 2000. (27) The calculation equations are listed as...

The caption:

The caption:

The caption:

The caption:

The caption:

The caption:
According to text in the paper China does not have enough dysprosium to meet its internal demand. (This is surprising to me.)
Several policy recommendations are made since China is both the largest producer and consumer of dysprosium.
One is to crack down on illegal mining - the isolation of lanthanides and their separation is very dirty chemistry, and when wildcatted, it is even worse.
Others are to increase recycling - although as noted above - recycling will not meet supply in a case of rising demand, rising demand being operative in the dysprosium supply.
I would imagine that most people do not think about this element. It's obscure. It is rather amazing however, to recognize how much depends on access to it, even in the case where the world comes to its senses and stops building wind turbines.
The light lanthanides are all fission products; the heavy ones (those past gadolinium) are not.
The Role of Copper Oxides in the Electrochemical Reduction of CO2 to C2 Carbon Fuels.
As I often point out, electricity is a thermodynamically degraded form of energy, and storing it as chemical energy degrades it even further. The caveat to this statement is that where electricity generation is a side product of another process involving high temperatures, such as the thermochemical production of hydrogen, or, as I recently discussed in this space, zero discharge supercritical water desalination. Under these circumstances, electricity, which may represent waste electricity if demand is low, can be utilized to increase the exergy of a system even if there is a thermodynamic loss associated with the conversion of electricity to chemical energy.
Here is the zero discharge desalination scheme I discussed fairly recently, in some detail, a situation in which electricity might be a side product of another process: The Energy Required to Supply California's Water with Zero Discharge Supercritical Desalination.
A great deal has been written about the electrochemical reduction of carbon dioxide to give various hydrocarbons, alcohols, aldehydes and organic acids - the latter most often formic acid. Recently I came across a nice review of the topic: Modeling Operando Electrochemical CO2 Reduction Federico Dattila, Ranga Rohit Seemakurthi, Yecheng Zhou, and Núria López Chemical Reviews 2022 122 (12), 11085-11130
Here is an intriguing graphic from the paper:

The caption:
Reference 129 is this one: Guiji Liu , Michelle Lee, Soonho Kwon, Guosong Zeng, Johanna Eichhorn, Aya K. Buckley, F. Dean Toste , William A. Goddard III , and Francesca M. Toma CO2 reduction on pure Cu produces only H2 after subsurface O is depleted: Theory and experiment, PNAS 118 (23) (2021) e2012649118
Here's a graphic from this paper:
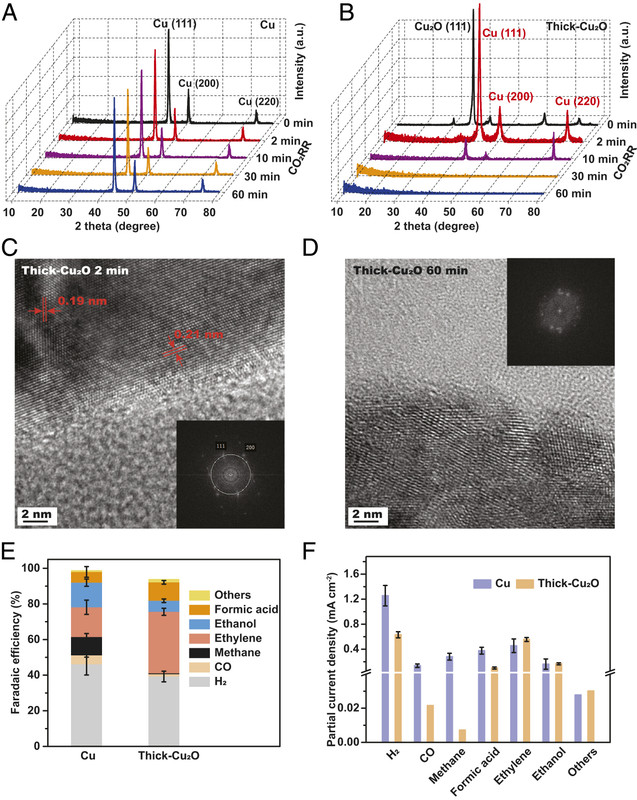
The caption:
It appears that copper (I) oxide is essential for producing hydrocarbons electrochemically and improves the yield over the production of hydrogen. Once the oxide is reduced, the product is more or less just hydrogen. The electrodes can be regenerated by simply exposing them to air, albeit for extended periods.
Regrettably I don't have much time to go into the details.
Hydrogen of course, can be converted to useful fuels via Fischer Tropsch chemistry (for petroleum like fuels) or fuels superior to petroleum, the best of which is dimethyl ether, a highly flexible and easily transportable fuel. It is not, however, despite decades of stupid rhetoric that still goes on and on and on and on, a useful consumer fuel: The infrastructure for so utilizing it would be expensive, unsustainable, and frankly dangerous. Hydrogen use should be limited to its already important realm as a useful captive intermediate in the chemical industry.
Direct reduction of carbonates to carbon fuels with water as the hydrogen donor is probably a good idea, again, only under the thermodynamic constraint that the electricity so utilized is a side product of other processes, represented "captured" exergy, available to be dispatched to grids on an "as needed basis."
Note that Faradaic efficiency is not the same as thermodynamic efficiency. Faradaic efficiency can be thought of as the fraction of electrons that end up in the product or in this case the products. The overall thermodynamic efficiency can be thought of as the product of the Faradaic efficiency and the Voltage efficiency, the latter representing the overvoltage required to drive the reaction. Essentially this is the extra energy to over come electrical resistance in the systems and represents energy lost as heat.
It will be interesting to see how nanostructured materials may be applied to further move these kinds of systems to ethylene to sequester carbon as polymeric material and to eliminate the use of dangerous fossil fuels.
Have a nice day tomorrow.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,515