NNadir
NNadir's JournalA Review Article On the Utilization of Carbon Dioxide.
As I often repeat, the failure of humanity to address the destruction of the planetary atmosphere will require future generations, should they prove capable of restoring whatever is left to restore of this planet, will require the removal of carbon dioxide from air, possibly via its removal from seawater.
This is a huge thermodynamic, and therefore engineering and energy challenge. It will require future generations to produce more energy than we now consume profligately, and with zero interest in the waste this energy production involves.
Of course in dealing with cleaning up our waste, future generations will be belabored and not enriched, but it's clear we couldn't care less about them, so it's their problem.
In this sense we are all Republicans, whether we acknowledge it or not; we care only about ourselves and have no interest in the welfare of other people. In this case, when I refer to "other people," I am referring to people who are now infants or children, and their children and infants, that is, all future generations.
Of course, it is not enough simply to remove carbon dioxide from the air; one must also have a place to put it.
The "place to put it" has often been imagined with the endless proposals of waste dumps euphemistically called "sequestration," although the word "dump" is appropriate. The current waste dump is of course, the atmosphere.
It turns out that another means of dealing with carbon dioxide would be to utilize it, which is a proposal to make it something other than "waste." Carbon dioxide is a currently utilized product industrially, although its use currently is nothing like the quantities dumped into the atmospheric waste dump, an amount on the order of 35 billion tons per year.
I think about these things a lot, and this is why I was pleased to go through the recent issue of Chemical Reviews, which was about sustainable chemistry.
An aside:
Recently in this space, in a post to which this post is a follow up, a comment of Vaclav Smil's (I wish people would embrace his clear thinking about energy, if not necessarily accepting his conclusions) about how refining of steel requires coal - I would argue that it need not do so forever, as I will briefly allude to below - that I partially repeat:
To answer the part I have put in bold above, my own definition of sustainability, if clearly not that of my contemporaries, would be a statement that each generation leaves for subsequent generations a planet that will afford ecosystem of the planet more or less in the same state into which they were born, and will allow the members of future generations the same level life style - including the opportunity to appreciate the beauty of the natural world - that the generation leaving enjoyed.
And no, the construction of millions of wind turbines that will be rotting hulks 30 years after construction, and millions of solar cells that will be electronic waste in thirty years won't cut it, any more than spent fracking fields - on which the fantasy to the contrary has depended, is depending and will always depend - leaching radioactive and chemically contaminated flow back water for centuries will cut it.
The paper I will discuss is this one: Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment (Leitner et al, Chem. Rev., 2018, 118 (2), pp 434–504)
The authors are German, and well they should consider this point, since the official and in my opinion extremely ignorant national German energy policy is to put lipstick on the pig of long term fossil fuel dependence which they are clearly interested in entrenching forever, or at least until every trace of fossil fuel waste that can be generated has been stuffed into the atmosphere and oceans, neither of which clearly can take it anymore without severe changes to their stability.
The paper's opening graphic, which I believe is accessible from the abstract is this one:

It is slightly inaccurate, since it seems to show a requirement for free isolated hydrogen, which is actually not necessary, although hydrogen might be present in many - maybe in most - schemes as a captive intermediate.
There are ways that carbon might be converted into products that do not depend on hydrogen, as I will describe briefly below.
"Life Cycle Analysis," "LCA," is an increasingly important discipline in evaluating the "sustainability" of a particular energy practice, but it is information dependent inasmuch as it depends on intimate knowledge of industrial processes. It is important to consider that it is also necessarily subjective.
For example it might matter to me if formerly pristine deserts are strewn with rotting metal thirty years from now as represented by abandoned wind farms, but it is not clear that if you establish the criteria as being "loss to human life" as being the only criteria that matters, the rotting metal abandoned wind farms will not be as important - although the production of the steel in them will have lead to losses of human life in the generation that built them because modern steel production always utilizes coke which is almost always made by heating coal.
Here is another graphic from the internal text of the paper that puts things in life cycle terms:

Here is the caption for that graphic:
Reference 117, Life cycle assessment of polyols for polyurethane production using CO2 as feedstock: insights from an industrial case study (Barlow and Assen, Green Chem., 2014, 16, 3272 does not contain the graphic immediately above, although the abstract shows a graphic present in the paper itself shows propylene oxide reacting with carbon dioxide to give a polymer. (Not shown is the solvent, which is DMC, dimethyl carbonate, which can also be made from carbon dioxide, as the review article discusses at length.)
Reference 117 is about a polycarbonate, a type of plastic which in this case is utilized to produce another polymer, polyurethane, albeit using a product obtained from dangerous fossil fuels, toluene isocyanate. (cf Green Chem, 2016, 16, 1865)
Returning however to the graphic above, note that it is an octagonal representation with lines from each of the vertices to the center representing a "reference case" for an environmental impact, and that the "global warming" impact is merely reduced, by less than 20%, not eliminated.
In reference 117 the process is described as capturing carbon dioxide from lignite coal burned in Germany's lignite coal plant at Niederaussem which is not being phased out like Germany's nuclear plants - all to be phased out - that do not require fossil fuels and which do not dump fossil fuel waste into the atmosphere.
Moreover, it's not clear that the carbon dioxide to make the polymer will never be added to the atmosphere, and quite possibly the whole enterprise, as described, is lipstick on the coal pig.
This said, were the carbon dioxide obtained from the removal of carbon dioxide from the atmosphere by carbon dioxide free means - which in my view can only be represented by nuclear energy, the following graphic from the review, a representation of the "savings" (with respect to direct dangerous fossil fuel waste dumping) would have a different form:

To wit, the denominator in the unit for the ordinate would disappear, since no carbon dioxide would be produced to make the polymer. To the extent that the polymer were recycled (albeit requiring an investment of energy) it would be possible to entirely close the carbon dioxide cycle, or at least minimize by a factor of perhaps 90% as opposed to "less than 20%."
(Don't you love "percent talk?" I actually don't, since it's most often used, in particular in the misrepresentation of the solar and wind industries as significant - which they are not when compared to the rapidly increasing use of dangerous fossil fuels, to perpetuate a lie rather than to describe the truth.)
Now let's turn to some chemistry, with this graphic from the review:

Reaction set (a) is lipstick on the methane (dangerous natural gas) pig, and refers to the mistaken impression that hydrogen is a "green" or "clean" fuel. This reaction - and it's coal based analogue - is, by the way, the means with almost 99% of the hydrogen industrially produced on the planet, by reformation, and not by electrolysis, the stupid exercise on the Norwegian Island of Utsira notwithstanding.
Reaction (b) is the partial reversal of reaction (a) and is known as the "water gas shift" reaction.
Reaction (c) is part of the international pipe dream about the wind and solar industries; it is extremely thermodynamically inefficient and the dream of expanding it to meaningful levels using technologies that have never proved meaningful, solar and wind, is in fact simply a measure of denial as pernicious as the level of denial practiced by the idiots in the Republican party.
Reaction (d) is of some interest depending on whence the high temperatures required come; provided by nuclear means, they can significantly improve the thermodynamic nightmare of electrolysis; produced by the solar thermal day dream - that industrially is environmentally destructive and totally dependent on supplemental dangerous gas as well as exorbitant in cost - it's just more garbage thinking.
Reaction set (e) is however quite interesting if only represented by loose schematic, non-stoichiometric carbon dioxide and water splitting.
In the modern scientific literature, this set (e) is almost always described in terms of "solar thermal" schemes. These do not work and will not work for the simple reason that batch processes are always more expensive and always dirtier than continuous processes, a fact that should be familiar to any well educated chemical engineer.
Historically these processes were often described by nuclear systems, and the most famous thermochemical hydrogen cycle, the sulfur iodine cycle, was that which was pushed by the General Atomics company, a company that proposed to build HTGC reactors with a helium working fluid Brayton cycle which would, in theory cogenerate hydrogen by splitting water.
The sulfur iodine cycle is not represented by the series in equations (e), nor should it be, since reactions (e) can produce either hydrogen or carbon monoxide, the latter being utilized by the use of reactions in set (a) to produce hydrogen without dangerous fossil fuels.
The GA HTGC reactors proved to be economic failures, since the attempt to build them - a few were built but didn't operate all that long - was before its time: The successful production of sustainable refractories advanced in the 1960's and 1970s in connection with the Space Program as a side product of that noble enterprise.
It is worth noting by the way, the General Atomics is now the site of one of two research nuclear fuels reactors in the United States, the other being at the Princeton Plasma Physics lab outside of Princeton, NJ.
If fusion ever became a practical form of energy - I doubt it will be significant in the lifetime of anyone now living, any more than solar or wind energy will be - all the reactions in set (e) would be practically and moreover sustainably driven.
About CO, carbon monoxide: Methane is often incorrectly thought to be "clean burning" because it produces low particulates, lacking the carbon-carbon bonds found in the admittedly dirtier fuels petroleum and coal.
The oxidation of methane does not, however, only produce the dangerous fossil fuel waste carbon dioxde since it is almost always the case that the oxidation is incomplete, and one incomplete product of said oxidation is carbon monoxide. Indeed the industrial process for producing hydrogen does this deliberately, partially oxidize methane.
Carbon monoxide however is not thermodynamically stable at all temperatures, it in fact exists in equilibrium with pure elemental carbon and carbon dioxide. This is known as the Boudouard equilibrium:
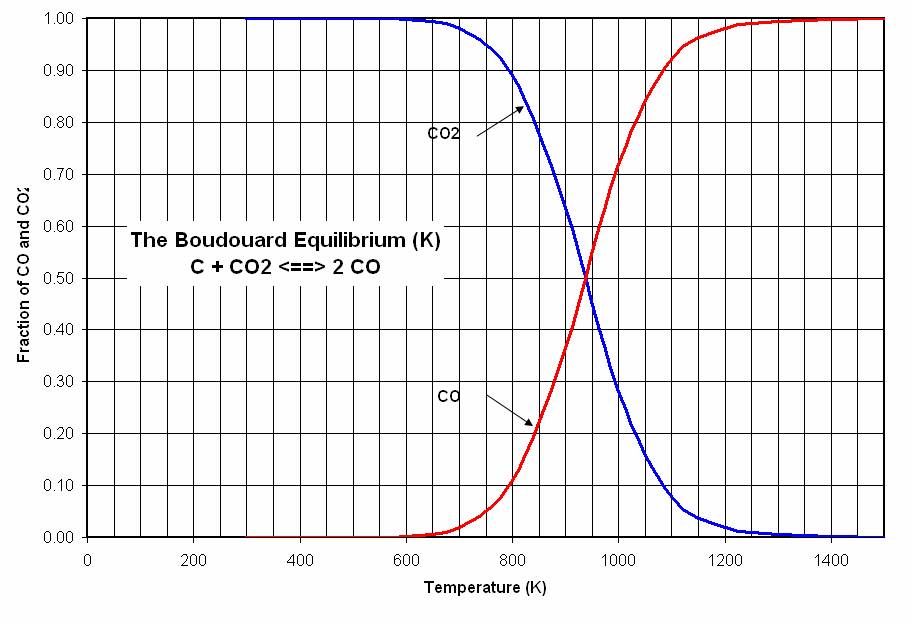
The Boudouard equilibrium shows that it is possible to obtain carbon from carbon dioxide, should one split carbon dioxide by reactions like those in reaction series (e), isolate carbon monoxide resulting from it, and then drive the Boudouard equilibrium to carbon by removing carbon dioxide by simple thermally reversible chemical means.
One of the tools for producing steel is carbon, whether the steel is structural steel in skyscrapers, or cars, or bridges or for that matter the quixotic enterprise of building windmills that pass, inappropriately, for energy decency in these times.
Theoretically at least, it may not thus be true that in order to make steel, one needs to mine coal.
And to the extent that metal carbides are used as important materials, and to the extent that other carbon based materials like those highly involved in modern nanotechnologies for just one example they represent utilized carbon that is not in the atmosphere.
A great deal of the review article covers the preparation of motor fuels from carbon dioxide. These are in general dependent on generating hydrogen, although some of the work covers electrolytic means of reduction, for example the electrolytic reduction of carbon dioxide to methanol, formaldehyde, formic acid and even alkanes.
Hydrogenation produces - I suspect with far greater thermodynamic efficiency - fuels like DMC, dimethyl carbonate, and the wonder fuel dimethyl ether, which for my money is the best energy storage material possible, far superior to hydrogen itself.
By reactions like those in set (e) above, all of these things can be accomplished by nuclear energy.
Is this easy? No, it isn't. There's no reason to be as glib as the failed "solar will save us" and "wind will save us" nonsense rhetoirc one hears all the time.
However if we are having a sustainable world, we are a long way away, and as everything we've done has failed, it's to try another way.
Below are some additional graphics from the review, which, being a review article discussing other papers, may or may not be broadly applicable. It is worth noting that many publications do not discuss nuclear energy, because nuclear energy is subject to broad public, if ignorant, approbation, and public approbation and attitudes do in fact, rightly or wrongly, effect the issuance of the grant system that supports our science.
A big graphic of the water gas equilibrium:

This graphic refers to "DRM" or dry reforming of methane, in which the dangerous fossil fuel natural gas is oxidized using CO2 rather than oxygen, a half-assed approach to eliminating carbon dioxide waste, but still an improvement in the efficiency of its use.:

The caption:
From my perspective, it is not enough to reduce carbon dioxide dumping, but it is essential to not dump it at all.
Here's a graphic representing the catalysts for "DRM:"

Here's a big blow up of the equations for metal based thermochemical carbon dioxide and water splitting. Metals included in these schemes are typically, if one wanders around the literature, iron, cerium, and (albeit not represented by the equations here, tin. There are other examples.

This graphic is a schematic of high temperature and low temperature electrolysis of water and carbon dioxide, performed in some cases synergistically.

This graphic refers to the production of the hydrogen storage compound formic acid, and regrettably refers to the use of dangerous fossil fuels, and is thus not a fossil fuel elimination scheme so much as a reduction scheme, and therefore in my personal view, not sustainable.

Some text from the review - there's lots of text - that describes an interesting and beautiful formic acid production scheme, albeit one that still needs work:
Whatever the limitations of the schemes found in this review, if you can find access, the review is worth a read.
Have a pleasant Sunday evening.
Landmines in the Republican War on the Middle Class: Home Equity Loan Interest No Longer Deductible.
My accountant sent out a notice that if you have a home equity type loan on your home, you will no longer be able to deduct your interest.
The money raised on the backs of the middle class in this particular example will go into a slush fund for the Trumps, the Kushners, the Wynns, the people who own Paul Ryan and Mitch McConnel and other people who couldn't care less about how you, for one example, put your kids through college.
If you're in this situation, you're going to have to pay your bank to refinance.
It's a word of warning. It could cost you tens of thousands of dollars if you're in this situation.
The Energy Requirement of Metal Processing and the Nuclear Option.
For my money, the most interesting, and possibly the most challenging thinker on the topic of energy in our age is Vaclav Smil.
I first became aware of him when I was thinking about the subject of catalytic nitrogen fixation and kept finding references in scientific papers on modern development of catalysts to his outstanding book on the history and development of this process on which our food supply is now entirely dependent, Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production.
I have read few books as thought provoking as this.
It is only possible to go through a small subset of Smil's writings and still have a life, but to the extent I have over the years, and whenever I have, I've been impressed with his vast knowledge of industrial processes, their interface with energy, and his associated stark realism, and if nothing else, they are stark.
Recently while reflecting on Richard Feynman's 1959 lecture, "There's Room at the Bottom" which predicted the information age and the nanotechnology age which has come to pass, I was inspired to refer to the book where I first encountered it - it's in the appendix - the Second Edition of Bradley Fahlman's wonderful text Materials Chemistry - and I found myself wandering to Chapter 3 in the book which is entitled "Metals."
Reading through sections of it I was reminded of one of Smil's writings that has really troubled me for years and challenged my technological thinking - and by the way I don't always agree with Smil but one has to think very deeply if one wants to seriously disagree with many of his profound insights - specifically, this one from 2009: The Iron Age & Coal-based Coke: A Neglected Case of Fossil-fuel Dependence.
(It comes from a "Free Market" blog, and I assure you that I am in no way a "free marketeer," since I am more inclined to think about sustainability, the very long term, as opposed to the psychology of our short term amoral generation of fools and "economic realities" that some people put forth, usually as I encounter them with the fraudulent statements that "solar energy is cheaper than grid energy," or "renewables are the fastest growing source of energy capacity." )
However much Smil and I may disagree on political economics however, I am certainly not in the mainstream of my political party, the Democratic Party - inasmuch as I think that the magical thinking that betting the future of the planetary atmosphere on wind and solar energy is at best wasteful and at worst tragic - I certainly agree with the introduction of his writing on the iron age:
High regard for facts and low regard for wishful thinking has forced me to deal repeatedly with many energy illusions–if not outright delusions–and to point out many complications and difficulties to be encountered during an inevitably lengthy transition from an overwhelmingly fossil-fueled world to economies drawing a substantial share of their primary energies from renewable sources.
I personally believe that the only sustainable form of energy is the cleanest and safest form of energy, nuclear energy. This is not, by the way, a statement that nuclear energy is without risk or that it is or has proved to be always harmless - clearly it hasn't - but my words contain the relational suffix "-est," cleanest, safest. It is merely a statement that nuclear energy is superior to all other options in energy.
Smil's point in the referenced article is that we must have coal because we must have steel.
I, by contrast, have convinced myself that nuclear energy should do everything. Am I engaged in the wishful thinking about which Smil is ever ready to challenge.
Steel...steel...steel...
If you want to get a feel for what's involved in steel, you should head out to Bethlehem, Pennsylvania and tour the steel stacks from the abandoned post industrial facility that used to be the chief plant for the defunct company Bethlehem Steel. The city of Bethlehem was left with this rotting hulk, and with wonderful creativity managed to turn it into a sort of industrial museum that also functions as an arts and music center. One can walk along a catwalk along side the towering retorts with nice little posters on industrial history, including comments on the immigrants who came to work there, and see where the steel for the Empire State Building, the Golden Gate Bridge, and the overwhelming majority of the "liberty ships" that won World War II was made.
It's a worthy afternoon, and if you go in summer, you can catch a nice evening concert on the surrounding grounds.
From Fahlman's book, on the subject of steel processing:
1. 500–600C: 1.Hematite (Fe2O3)
2. 600–900C: Magnetite (Fe3O4)
3. 900–1,100_C: Wustite (FeO)
4. >1,100_C : FeO0.5
Since iron ore is largely comprised of aluminosilicate minerals, a byproduct is also formed within the blast furnace, known as slag (ca. 30–40 wt.% SiO2, 5–10 wt.% Al2O3, 35–45 wt.% CaO, 5–15 wt.% MgO, and 5–10 wt.% CaS).
It should be noted that it takes 6–8 h for the native iron ore to descend toward the bottom of the blast furnace, but only ca. 8 s for the pre-heated air to reach the top of the furnace. Oftentimes, a fused solid known as sinter is also added to the blast furnace, which is comprised of fine particulates of iron ore, coke, limestone and other steel plant waste materials that contain iron. The reducing agent within the blast furnace (coke) is comprised of 90–93% carbon, and is formed by heating coal to remove the volatile components such as oil and tar. The coke is ignited at the bottom of the blast furnace immediately upon contact with the air blast; since there is excess carbon in the furnace, the active reducing species is CO rather than CO2.
You get the idea...
Further text refers to the removal of sulfur from the pig iron, using calcium oxide, "burnt lime," itself requiring a huge investment in heat for manufacture at 1400C and then transferring the molten metal using a ladle to the "BOF," the basic oxygen furnace, where the metal is treated with a supersonic jet of pure oxygen with the temperature rising to 1700C to remove phosphorous, carbon, manganese and silicon.
Later there's reference to the use of pure argon gas for alloying purposes. The purification of argon, which represents about 1% of the atmosphere is also an extremely energy intensive process.
There's quite a bit in this text, including some wonderful pictures of a steel plant in Dearborn, Michigan, Sevarstar Steel, then the American subsidiary of a Russian company that as of today has been sold to an American company AK Steel. The chapter also contains wonderful pictures of other industrial facilities to process other metals. If you can find access to this book, and you're at all interested on a profound level on the subject of sustainability, it's almost an essential read, along with reading Smil.
Smil's argument is that there is simply not enough carbon available in biomass to replace coal.
I would counter that there is certainly enough carbon in the atmosphere to replace coal however, if one can reduce it.
In the future in this space, I hope to write a commentary on a paper in the current issue of Chemical Reviews which is dedicated to "Sustainable Chemistry" to discuss carbon dioxide/water splitting thermochemical cycles.
Is it conceivable that we can replace carbon from coal for processing iron with nuclear energy? I believe it is. Is it easy to do so? Probably not. My impression is, however, that irrespective of overcoming the unsustainable temporal psychology of "free markets" we have a moral obligation to all future generations to explore this.
My insomnia, under which I write this piece is finally being overcome, and I'm going for a nap. If interested, stay tuned. I'll leave you with a link to what Vaclav Smil sees when he sees a wind turbine, with a cool graphic dripping with oil:
What I See When I See a Wind Turbine
I see something different, something worse than Smil sees - the future unless we change our minds - but neither of us are fond of wind energy, which is not clean, not "green" and not sustainable.
Have a pleasant weekend.
Porous Rhodium Copper Nanospheres.
In my electronic files, I have a copy of the Second Edition of Bradley Fahlman's wonderful text Materials Chemistry - a Third Edition either has, or is about to be released - which in its Appendix B reproduces a December 1959 lecture by Richard Feynman entitled "There's Room at the Bottom," in which he discusses a putative world in which it is possible to print the Encyclopedia Britannica on the head of a pin.
His speech began like this:
As soon as I mention this, people tell me about miniaturization, and how far it has progressed today. They tell me about electricmotors that are the size of the nail on your small finger. And there is a device on the market, they tell me, by which you can write the Lord’s Prayer on the head of a pin. But that’s nothing; that’s the most primitive, halting step in the direction I intend to discuss. It is a staggeringly small world that is below. In the year 2000, when they look back at this age, they will wonder why it was not until the year 1960 that anybody began seriously to move in this direction. Why cannot we write the entire 24 volumes of the Encyclopedia Brittanica on the head of a pin?
Let’s see what would be involved...
Later he continues in what seemed to him to be a perfectly reasonable possibility, although I'm not sure that everyone in his audience found it believable:
How much space would it take? It would take, of course, the area of about a million pinheads because, instead of there being just the 24 volumes of the Encyclopaedia, there are 24 million volumes. The million pinheads can be put in a square of a thousand pins on a side, or an area of about 3 square yards. That is to say, the silica replica with the paper-thin backing of plastic, with which we have made the copies, with all this information, is on an area of approximately the size of 35 pages of the Encyclopaedia. That is about half as many pages as there are in this magazine. All of the information which all of mankind has every recorded in books can be carried around in a pamphlet in your hand – and not written in code, but a simple reproduction of the original pictures, engravings, and everything else on a small scale without loss of resolution.
What would our librarian at Caltech say, as she runs all over from one building to another, if I tell her that, 10 years from now, all of the information that she is struggling to keep track of – 120,000 volumes, stacked from the floor to the ceiling, drawers full of cards, storage rooms full of the older books – can be kept on just one library card! When the University of Brazil, for example, finds that their library is burned, we can send them a copy of every book in our library by striking off a copy from the master plate in a few hours and mailing it in an envelope no bigger or heavier than any other ordinary air mail letter.
Now, the name of this talk is ‘There is Plenty of Room at the Bottom’ – not just ‘There is Room at the Bottom.’ What I have demonstrated is that there is room – that you can decrease the size of things in a practical way. I now want to show that there is plenty of room. I will not now discuss how we are going to do it, but only what is possible in principle – in other words, what is possible according to the laws of physics. I am not inventing anti-gravity, which is possible someday only if the laws are not what we think. I am telling you what could be done if the laws are what we think; we are not doing it simply because we haven’t yet gotten around to it.
1959...Remarkable...completely and totally remarkable.
As we all know the world that Feynman predicted in 1959 has come to pass. I can easily carry Fahlman's book, and a thousand books like it, plus thousands of copies of papers, photographs in my pocket on a thumb drive, which is something I do frequently.
What is more remarkable is that it is how possible to actually see things at an atomic scale. I had a nice tour with my son of the materials science department that he ultimately went to school, and the nice graduate student who conducted the tour took us to see a whole bunch of different microscopes, including an tunneling electron microscope where he displayed a photography of, um, atoms.
Whatever.
This is a brief note about a nanotechnical approach to utilizing vanishing resources more carefully.
I'm sure I've posted this periodic table in this space before, which shows the "critical elements" that are expected to run out, at least in traditionally processed ores accessible at low prices and utilized using current technology:
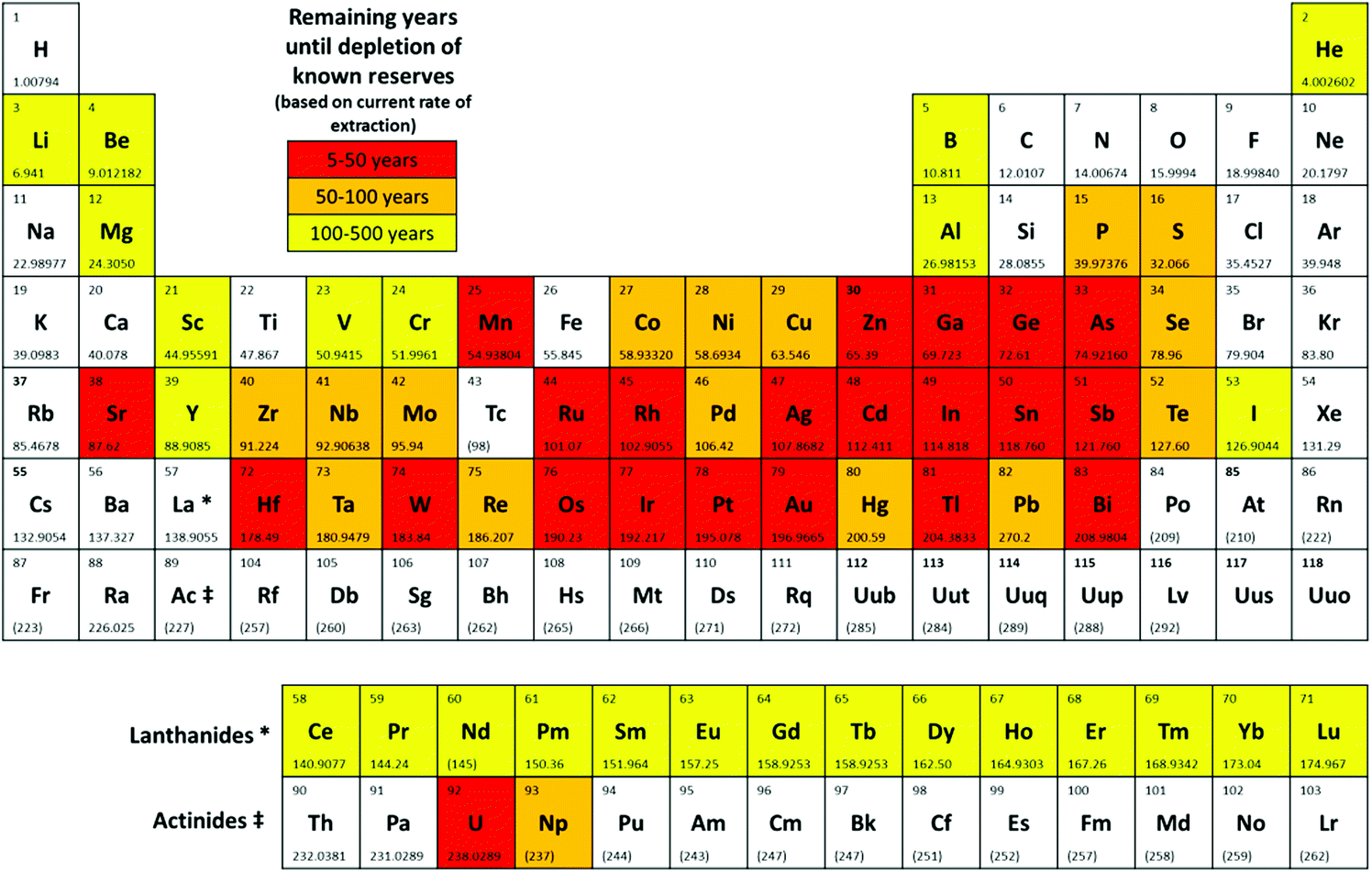
One may quibble a bit on the data this table represents - I have argued that because of its high energy density supplies of uranium are inexhaustible, for example - but I'm quite sure, the regrettable circumstance of "peak oil" not having come to pass - that in most cases the table explicates a serious threat to future generations; that many elements will become inaccessible.
One of the elements in red, element 45, rhodium is the one I'd like to discuss by pointing to a paper I came across today (and put on my thumb drive), this one: Mesoporous Bimetallic RhCu Alloy Nanospheres Using a Sophisticated Soft-Templating Strategy (Yamauchi et al, Chem. Mater., 2018, 30 (2), pp 428–435)
Rhodium is an important catalyst; it also serves as a minor constituent of alloys of profound technological importance.
According to another paper from a few years back (Electrochemical behavior of rhodium(III) in 1-butyl-3-methylimidazolium chloride ionic liquid, Srinivasan et al, Electrochimica Acta Volume 53, Issue 6, 15 February 2008, Pages 2794-2801) supplies of this element from terrestrial ores will actually be lower than the quantities available for isolation from used nuclear fuel.
The Yamuchi paper has a nice description of some of the important uses of rhodium catalysts:
The authors explore then, the Feynman solution, which is to make nanoparticles consisting of a copper rhodium allow in porous perforated spheres:
Scheme 1 as a graphic:

The caption:
A scanning electron microscope picture of the resulting balls:

The caption:
A tunneling electron microscope picture of them, touching on the atomic scale:

The caption:
Rhodium is a high melting metal; its alloy with copper melts lower. The authors suggest that this morphology actually overcomes the tendency for other copper rhodium systems to agglomerate, thus reducing the catalytic efficiency and lifetime.
Cool I think. This sort of thing should extend rhodium resources, at least until the human race comes to its senses and begins to utilize the valuable materials in used nuclear fuel.
Have a pleasant "hump day" tomorrow.
In my earnest opinion, a little demeaning is appropriate.
People expect that they are speaking to another idiot when they pull out stuff like their "uranium miner" fetish, which is, to be perfectly honest, Trumpian in its scale.
Between 1950 and 2005, the period covered in my post, according to the US Department of Labor, 16,702 people died in coal mining accidents.
It's pretty funny when nominally liberal people start lecturing all the time on "markets forces" and "economic realities."
The economics of gas and coal and oil are all dependent on the dubious practice of not including their well known and well understood external costs, which are the costs in human health and human life.
Suppose we valued a human life at $10,000 - a figure that is obviously arbitrarily low. This means the economic cost of seven million deaths per year is 70 billion dollars a year. If we raise the figure to $100,000 for a life it's $700 billion a year, or 7 trillion every decade.
Please don't lecture me about markets and economics.
As it happens these calculations do not include the cost of dying. My father, a cigarette smoker, died from lung cancer. I watched him die. The cost of his struggle cost the government, his insurers, not to mention my stepmother, a little over $200,000.
Again, please don't lecture me about markets and economics.
The all sacred "market" doesn't level the "playing field." It allows one subset of people to kill a larger subset of people without any attempt to account for the economic, never mind the moral cost.
And excuse me if I don't necessarily agree that I need to understand that "most people" care deeply for humanity and the planet.
If they cared deeply, they wouldn't make so many glib and poorly thought our remarks as I see all the time on the topic of energy and its impact on humanity. Please note that I care not just for people killed by energy technology, but also those people who die from a lack of access to energy.
The average continuous power consumption of human beings on this planet is about 2500 watts, roughly a quarter of the power consumption of the average American.
I have argued that if we were - in a campaign to eliminate human poverty - to double this average to 5,000 watts, and provide that power from the fission of plutonium, each person living to the age of 100 would responsible for the fissioning of about 100 grams of material in their entire lifetime.
Current Energy Demand; Ethical Energy Demand; Depleted Uranium and the Centuries to Come
I've heard this platitude about stones and the stone age many times; I believe it came from a Saudi oil minister.
It's insipid, and frankly doesn't mean anything at all.
The technology to eliminate fossil fuels in their entirety was invented by some of the finest minds ever to grace this planet. It's operated despite opposition from people who are rather lazy, uninformed and engaged in poor thinking for half a century.
It hasn't been allowed to advance to do what it might have done, because, for one example, people evoke fetishes about uranium miners to the exclusion of all other energy related deaths.
This doesn't strike me as "caring." It strikes me as indifference.
Do you have any idea of how many of these miners, subject of so many fetishes, there were?
I do.
I wrote about them here: Sustaining the Wind Part 3 – Is Uranium Exhaustible? The references to the scientific papers from which my discussion excerpted below comes can be found in the links I provided when I submitted the work to Dr. Brooks for publication on his website.
I showed, citing something called the "scientific literature" that about 63 miners died earlier than expected in a control population from lung cancer, while fewer cancer deaths among the miners took place than expected in a control population from all other cancers. Many miners died from other causes - like automobile and other accidents
Right now, on this planet, not that our fetishizing anti-nuke selective attention crowd gives a shit about anyone who dies unless uranium is involved, 7 million people die every year from air pollution, about half from the combustion of of "renewable biomass," and the other half from dangerous fossil fuel waste.
A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010 (Lancet 2012, 380, 2224–60: For air pollution mortality figures see Table 3, page 2238 and the text on page 2240.)
This works out to about 19,000 people per day, or around 800 every hour.
If a little fewr 70 uranium miners died from premature lung cancers, and there's good evidence they did, this amounts to about 13 minutes worth of air pollution deaths.
Like all anti-nuke fetishes, it's purely selective attention.
I note that nobody gives a shit when native Americans or anyone else dies in a coal mining accident, or black lung disease, or a gas explosion, but tons of books have been written about the uranium miners.
Now the Dine miners didn't die instantly, as shown that many were still alive well into old age, in contrast to say, people killed in a coal mine explosion, deaths numbering the thousands.
By contrast, most of the people who die because assholes carry on about the risks of nuclear energy while allowing the growth of fossil fuel combustion to kill more people each year than in the previous year from air pollution are under the age of 5.
But the uranium miners and their "suffering" matters, and the five year old's suffering is trivial.
Excuse me if this mentality disgusts me.
The relevant excerpt my analysis is here:
As I prepared this work, I took some time to wander around the stacks of the Firestone Library at Princeton University where, within a few minutes, without too much effort, I was able to assemble a small pile of books[50] on the terrible case of the Dine (Navajo) uranium miners who worked in the mid-20th century, resulting in higher rates of lung cancer than the general population. The general theme of these books if one leafs through them is this: In the late 1940’s mysterious people, military syndics vaguely involved with secret US government activities show up on the Dine (Navajo) Reservation in the “Four Corners” region of the United States, knowing that uranium is “dangerous” and/or “deadly” to convince naïve and uneducated Dine (Navajos) to dig the “dangerous ore” while concealing its true “deadly” nature. The uranium ends up killing many of the miners, thus furthering the long American history of genocide against the Native American peoples. There is a conspiratorial air to all of it; it begins, in these accounts, with the cold warrior American military drive to produce nuclear arms and then is enthusiastically taken up by the “evil” and “venal” conspirators who foist the “crime” of nuclear energy on an unsuspecting American public, this while killing even more innocent Native Americans...
...Still, one wonders, was hiring Dine/Navajo uranium miners yet another case of official deliberate racism as the pile of books in the Firestone library strongly implied?
Really?
A publication[51] in 2009 evaluated the cause of deaths among uranium miners on the Colorado Plateau and represented a follow up of a study of the health of these miners, 4,137 of them, of whom 3,358 were “white” (Caucasian) and 779 of whom were “non-white.” Of the 779 “non-white” we are told that 99% of them were “American Indians,” i.e. Native Americans. We may also read that the median year of birth for these miners, white and Native American, was 1922, meaning that a miner born in the median year would have been 83 years old in 2005, the year to which the follow up was conducted. (The oldest miner in the data set was born in 1913; the youngest was born in 1931.) Of the miners who were evaluated, 2,428 of them had died at the time the study was conducted, 826 of whom died after 1990, when the median subject would have been 68 years old.
Let’s ignore the “white” people; they are irrelevant in these accounts.
Of the Native American miners, 536 died before 1990, and 280 died in the period between 1991and 2005, meaning that in 2005, only 13 survived. Of course, if none of the Native Americans had ever been in a mine of any kind, never mind uranium mines, this would have not rendered them immortal. (Let’s be clear no one writes pathos inspiring books about the Native American miners in the Kayenta or Black Mesa coal mines, both of which were operated on Native American reservations in the same general area as the uranium mines.) Thirty-two of the Native American uranium miners died in car crashes, 8 were murdered, 8 committed suicide, and 10 died from things like falling into a hole, or collision with an “object.” Fifty-four of the Native American uranium miners died from cancers that were not lung cancer. The “Standard Mortality Ratio,” or SMR for this number of cancer deaths that were not lung cancer was 0.85, with the 95% confidence level extending from 0.64 to 1.11. The “Standard Mortality Ratio” is the ratio, of course, the ratio between the number of deaths observed in the study population (in this case Native American Uranium Miners) to the number of deaths that would have been expected in a control population. At an SMR of 0.85, thus 54 deaths is (54/.085) – 54 = -10. Ten fewer Native American uranium miners died from “cancers other than lung cancer” than would have been expected in a population of that size. At the lower 95% confidence limit SMR, 0.64, the number would be 31 fewer deaths from “cancers other than lung cancer,” whereas at the higher limit SMR, 1.11, 5 additional deaths would have been recorded, compared with the general population.
Lung cancer, of course, tells a very different story. Ninety-two Native American uranium miners died of lung cancer. Sixty-three of these died before 1990; twenty-nine died after 1990. The SMR for the population that died in the former case was 3.18, for the former 3.27. This means the expected number of deaths would have been expected in the former case was 20, in the latter case, 9. Thus the excess lung cancer deaths among Native American uranium miners was 92 – (20 +9) = 63.
Thanks for your comment. It pretty much - once again, for the umpteenth time - lets me know all about the ethical universe in which anti-nukes live, a fetishist world where anything and everything is allowed to kill in massive amounts just to assure us that no one ever dies from radiation.
Please excuse me if I find this indifference to human life appalling.
Enjoy the coming work week.
Donald Trump, the Ig Nobel Prize, and the Dunning - Kruger Effect.
One of the many joys of living in the Princeton, NJ area is being near the Princeton Plasma Physics labs which every winter hosts the Ronald Hatcher Science on Saturday Lecture Series every winter.
I started going to it many years ago with my sons to stimulate their interest in science, way back when it was actually hosted by Ron Hatcher, a PPPL engineer, who regrettably died young, whereupon the lecture series took his name.
My sons are now both in college, and both working very hard, so they no rarely come with me any more, and because my wife is very busy with her work, I now go alone if I have time.
Ron Hatcher hosting a talk:
 ?itok=tYkjYzky&p2cy2v
?itok=tYkjYzky&p2cy2v
The series is now hosted by a PPPL outreach scientist, Andrew Zwicker whose part time job is being the Democratic New Jersey Assemblyman from the 16th Assembly district. (Not my district by the way.) Although I personally disagree with Dr. Zwicker on many issues about science and technology - he often evokes for me of the famous Upton Sinclair adage, " "It is difficult to get a man to understand something, when his salary depends upon his not understanding it." - he is a delightful human being and scientist, a real asset to the community who takes time away from his family to host the Science on Saturday lectures 8 weekends every winter, with wit and grace.
Sometimes I go to these lectures a little strung out when I intend to challenge the speaker on some topic or another. My sons always joke with me about "Voooonnnnn Hippel," who it turns out is a very gracious man, albeit a wrong headed man, who is a famous physicist at the Woodrow Wilson of Public and International Affairs, whose lecture was fine - about nuclear arms control - although his writings on other subjects are disturbing, at least to me.
Today's lecture was by Marc Abrahams, the founder and chief administrator of the Ig Nobel Prizes, and the editor of the Journal of Improbable Research which discusses papers that inspire laughter.
While I was generally familiar with the Ig Nobel Prizes, I'd rather thought that they were generally derisive, and in these times where science is under attack by the very Government that should be supporting it (and has, historically supported it) - the United States Government - being as thin skinned as I am, I was ready to be angry at this lecture, since I had it my head that the tone of the prizes is to make people think science is an over funded waste.
I mean one can imagine someone ridiculing the idea that studying where an electron might be in an atom was a scurrilous and silly idea if one were to hear about it in isolation, but in fact, it is part of the foundation of quantum mechanics.
Instead I was delighted by the Ig Nobel lecture.
First of all, the awards are not about ridicule - all winners are given the chance to anonymously decline the "prize," though few actually do - and the purpose of the prize is well, I'll let the Ignoble Prize people describe their purpose:
...and then make them think.
The prizes are hardly derisive, but they are fun and in fact, do make you think, as was obvious from the engagement of the audience during the question and answer period following the lecture.
Abrahams is a fascinating speaker as well as a credible comedian and he walked us through some of the research winning the awards, as well as some of the acceptance speeches of the generally wonderful winner/scientists from the scientists, including:
PHYSICS PRIZE [FRANCE, SINGAPORE, USA] — Marc-Antoine Fardin, for using fluid dynamics to probe the question "Can a Cat Be Both a Solid and a Liquid?"
ECONOMICS PRIZE [AUSTRALIA, USA] — Matthew Rockloff and Nancy Greer, for their experiments to see how contact with a live crocodile affects a person's willingness to gamble.
REFERENCE: "Never Smile at a Crocodile: Betting on Electronic Gaming Machines is Intensified by Reptile-Induced Arousal," Matthew J. Rockloff and Nancy Greer, Journal of Gambling Studies, vol. 26, no. 4, December 2010, pp. 571-81.
...and...
MEDICINE PRIZE [FRANCE, UK] — Jean-Pierre Royet, David Meunier, Nicolas Torquet, Anne-Marie Mouly, and Tao Jiang, for using advanced brain-scanning technology to measure the extent to which some people are disgusted by cheese.
REFERENCE: "The Neural Bases of Disgust for Cheese: An fMRI Study," Jean-Pierre Royet, David Meunier, Nicolas Torquet, Anne-Marie Mouly and Tao Jiang, Frontiers in Human Neuroscience, vol. 10, October 2016, article 511.
The full list of winners, along with links to their papers is found here: https://www.improbable.com/ig/winners/
But the high point of the lecture was a discussion, in the first few minutes of the lecture, of the 2000 winner of the Ig Nobel Prize in Psychology, Cornell Psychologists David Dunning and Justin Kruger for their paper: "Unskilled and Unaware of It: How Difficulties in Recognizing One's Own Incompetence Lead to Inflated Self-Assessments."
After briefly describing the paper, Abrahams...wait for it...flipped the slide to show a big picture of the Stupido in Chief Donald Trump wearing his dumbest idiot grin - the grin we have all come to know, regrettably, you know, the "real stable genius" grin.
The audience just exploded with laughter and applause, a great moment, a joyous moment.
Makes you think...
Here's the award winning paper: Unskilled and Unaware of It: How Difficulties in Recognizing One's Own Incompetence Lead to Inflated Self-Assessments (Journal of Personality and Social Psychology 1999, Vol. 77, No. 6. ] 121-1134)
Apparently the paper was right, and the asshole in the White House proves it.
The Prize is usually given, by the way, by real Nobel Laureates, and there is one winner of the Ig Nobel who also won the real Nobel, the Ig Nobel for a paper on levitating a frog using the magnetic properties of water, and the Nobel for his work on Graphene, Dr. Andrew Geim at the University of Manchester.
A fun day...a delightful day.
Have a nice Sunday.
A superion conductive material for sodium based batteries.
One hears a lot about batteries these days, usually in connection with the unfortunate fantasy that so called "renewable energy" will someday become a significant and clean for of energy; that all our problems will be solved if only we had great batteries to store our wind and solar energy that we imagine is already significant and cheap.
They haven't been; they are not; and they will not be significant and cheap, but no matter. (After 50 years of cheering, solar and wind do not produce 10 of the 576 exajoules humanity generates and consumes each year, as of 2016.)
In any case, I'm not so sure that batteries are a good idea in a purely environmental sense, since they require that humanity generates more energy than it already does:
A battery is a device that wastes energy owing to the second law of thermodynamics, but this said, there are many applications - we all use them - where they are desirable and in fact, essential, a fact that has nothing to do whether the source of electricity is relatively dirty - fossil fuels and to a lesser extent solar and wind - or clean, nuclear energy.
The best batteries in the world of course are currently lithium batteries, but there are reasons that they are not ideal, one being that lithium is an ion that is known to have profound biological effects, primarily neurological, and secondly - although I don't believe that it is as critical an issue - it is somewhat problematic to obtain and refine it.
A better element for economic and environmental reasons would be sodium, since it is readily available in vast quantities, is cheap, and is an essential component of all living things, and thus is non-toxic.
Thus it was interesting to encounter a paper on the path to making sodium batteries, moreover solid state sodium batteries, this paper: Na11Sn2PS12: a new solid state sodium superionic conductor (Nazar et al, Energy Environ. Sci., 2018, 11, 87-93)
In the introduction, the authors review the state of the art in solid phase batteries.
Germanium, Ge, is an element that is readily subject to depletion, even if lithium resources can be extended nearly indefinitely, at least in the case where one has sufficient energy to isolate it.
The authors then report an alternative, the sodium tin phosphorous sulfide battery which can be manufactured from earth abundant elements, as opposed to lithium and germanium.
Here is the (beautiful) structure of their sodium ion conducting material:
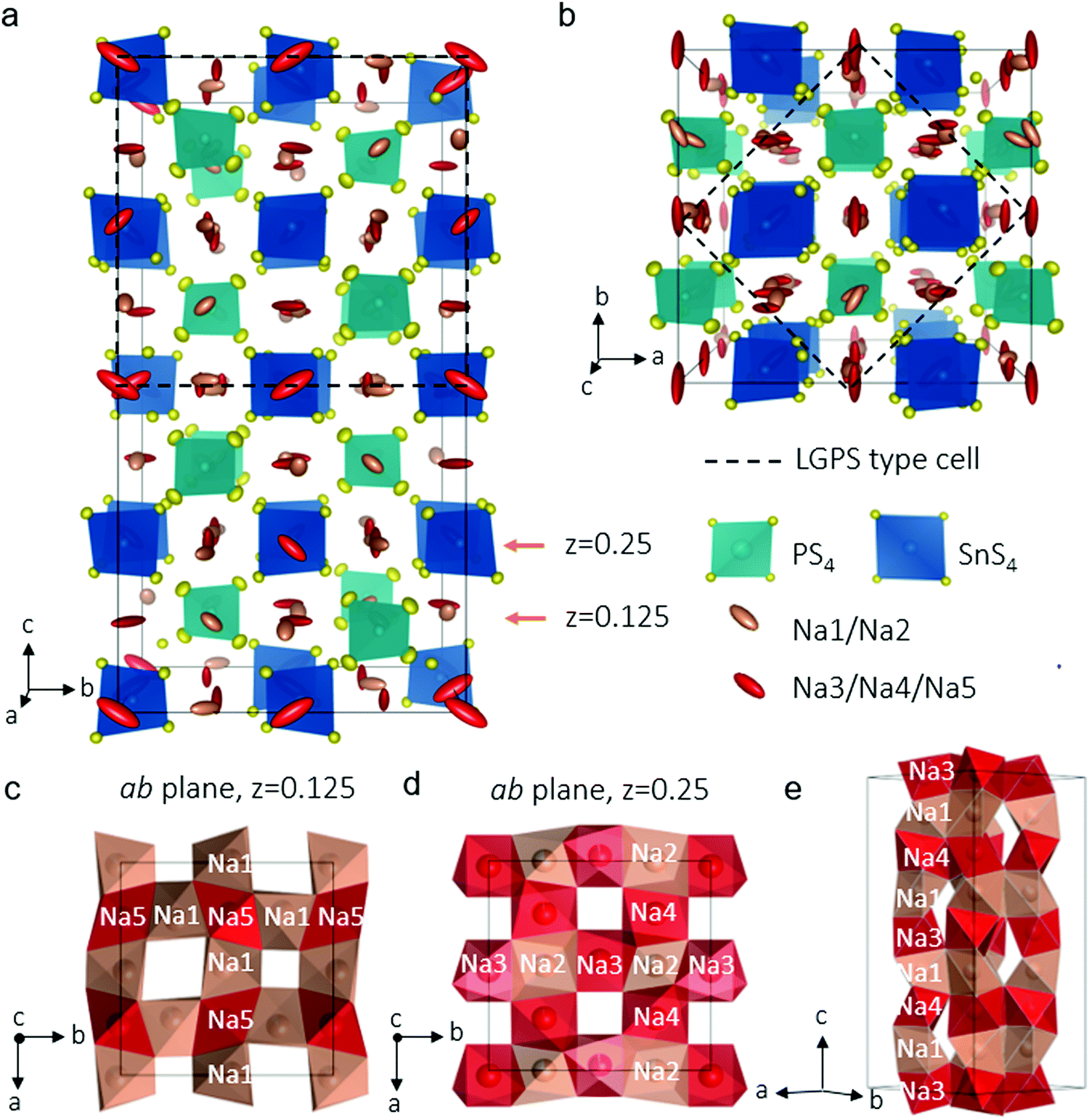
Here's the caption of the diagram:
There's some nice details on the synthesis of their ion conductor as well as measurements of its ion conductive properties, etc, and then some nice discussion of modeling of the structure via DFT (Density Functional Theory) calculations.
It's interesting and cool I think. I personally believe that sodium based batteries are a better idea than lithium batteries, but that's just my opinion.
Have a nice weekend.
3D barcodes: The Heuristics of Calculus and the Unrelated Density of Information In Cell Biology.
Abandoned by his alcoholic father, my father dropped out of school in the 8th grade to go to work - he shined shoes for a living until he joined the Navy in order to be sunk by the Germans, and shot at by the Japanese. He never went back to school, using his GI benefits not for an education - since it would have involved time to get a GED, and then go to college - but to buy the house in which I grew up.
His main interest in mathematics was to ask me to calculate the area of things when he worked in the woodshop he assembled in the basement, where he worked whenever he wasn't working 14 hour days packed with overtime so he could afford to buy me and my brother trinkets.
I was never able to teach him the 9th grade math required to calculate areas beyond squares and rectangles for himself, but he made stuff anyway and I helped him whenever he needed it.
All this said, the most important thing about buying a house for my father was the schools, and looking back for its time, I was educated in a pretty good school district, at least for its time. My house was a lower middle class neighborhood in an otherwise upper class professional school district. Before the age of the minicomputer it was one of the first schools to have any kind of computer - you put the hand receiver into a special holder to contact a main frame somewhere at some national lab - and one of the few schools around where students could take a calculus course before going to college.
These things are of course ordinary now: My son took three courses in calculus in high school, repeating the third one in his first year at his university to be sure that nothing was missing, only to report that taking the class was largely a waste of time except as a review. Like most children, too, he grew up using computers far more powerful than those used by the best engineers of my childhood and teen years, the NASA engineers who built the American space program that led to all the discoveries that now define our ordinary lives.
Nevertheless, looking back on my high school calculus education - where I was a "good" student, in the top ten percent in a school where everybody else's father was an engineer, a physician, a pilot, a Park Avenue lawyer or some such thing, although my father drove a forklift in a unionized warehouse - I realize that my mathematics education was extremely deficient in important ways.
Looking back, I realize I emerged with some very profound areas of ignorance, the most important being the belief - which no one corrected - that mathematics was just an abstraction, a cute curiosity of little practical import, except of course, to calculate the area of a table top so you knew how much wood to buy.
Seriously. And that was a "good" school.
I think it's a serious disadvantage - one that our culture should do everything to address and overcome, even our government is notable for its profound contempt for and hatred of poor people, unlike the Jesus they always prattle on about - for a child to grow up with uneducated, or poorly educated parents. (Look how stupid Donald Trump Jr. is, for example.) We waste time and resources, critical human resources, when we fail to value our teachers and fail to understand what they need to be trained to understand about the minds of children, and the stumbling blocks those children face.
When my boys were growing up, I made sure to discuss with them the concept of what a dimension is - I thought while growing up that a "dimension" was a fuzzy topic in some science fiction books where travelers from "other dimensions" traveled to our world - which is to say that a dimension is best regarded as a scalar value comprising a vector. One of the things my sons understood before going to high school was that three dimensional space as represented by the x, y, z co-ordinates is just an arbitrary way of translating other systems having multiple dimensions into easily visualized geometrical structures. My oldest son, the art student, confers with his brother, the engineering student about vectors while figuring out how to program web pages. It's ordinary for them, but it was in no way ordinary to me, even after I went to college.
This why it was such a pleasure for me to come across this very nice paper on cellular biology: Three-Dimensional Barcodes with Ultrahigh Encoding Capacities: A Flexible, Accurate, and Reproducible Encoding Strategy for Suspension Arrays (Si Lu, Ding Shengzi Zhang, Dan Wei, Ye Lin, Shunjia Zhang, Hao He, Xunbin Wei , Hongchen Gu, and Hong Xu, Chem. Mater., 2017, 29 (24), pp 10398–10408).
The authors - from Shanghai Jiao Tong University Affiliated Sixth Hospital, School of Biomedical Engineering, Shanghai Jiao Tong University - wrote in the abstract, for the reasons above, strike me, as a result of my upbringing, a beautiful statement to which I wish I'd been exposed when I was a child, to wit, this:
There you have it, three dimensions that are not really space related, with the possible exception of "size" which, in this case can be expressed as simple unidimensional scalar, the radius, since the particles are spheres.
Here is a picture, from the text of the paper, of the particles:

The caption of the picture is this:
The purpose of these highly dense information systems is described in the text of the paper:
Nonetheless, in multiplex assays, the number of target molecules that can be assessed in parallel is restricted to the number of distinguishable barcodes in flow cytometry. To date, numerous successful cases have been developed to enlarge the encoding capacity of barcodes based on two commonly used encoding fluorophores, organic dyes(12-17) or quantum dots (QDs).(18-27) The most classical dye-encoding system is the commercialized xMAP technology,(15-17) where orange and red dyes are loaded in microscaled beads at 10 different concentrations by the swelling method. Hence, an encoding capacity of 100 barcodes has been obtained via flow cytometry.
Flow cytometry is a technique where individual cells - they could be cancer cells, or cells designed to be pluripotent cells to restore or regrow damaged tissue (or even, someday, whole organs), or cells for some other purpose - are isolated on an individual level so that one can learn something about them, it could be the biochemistry of their surfaces, or information about their genome, or something about their general chemistry of biophysics.
The authors write about building a coding system of particles:
Here's the schematic of "Scheme 1"

The caption:
"DMB" refers to "dual-encoded magnetic barcodes."
There's some cool chemistry and biochemistry in the paper, both analytical and biochemical, and the authors write finally in their conclusion thus:
A cool paper out of China where - say what you will about the Chinese government - the government doesn't hate science as much as the people controlling our government do.
I hope you have a very pleasant weekend.
Are you going with me?
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,509