NNadir
NNadir's JournalCovid inflection in this year's CO2 accumulation data.
I check the Mauna Loa CO2 observatory figures every week, but by habit, I apparently don't pay attention to the annual graph which is below.
Almost every year, the graph is sinusoidal with each year's peak (usually in May) higher than the peak the previous year. (This is a function of our complete failure to address climate change.)
I didn't notice the "Covid Inflection" in the 2020 graphic until today, which appeared in the late February-Early March time frame:
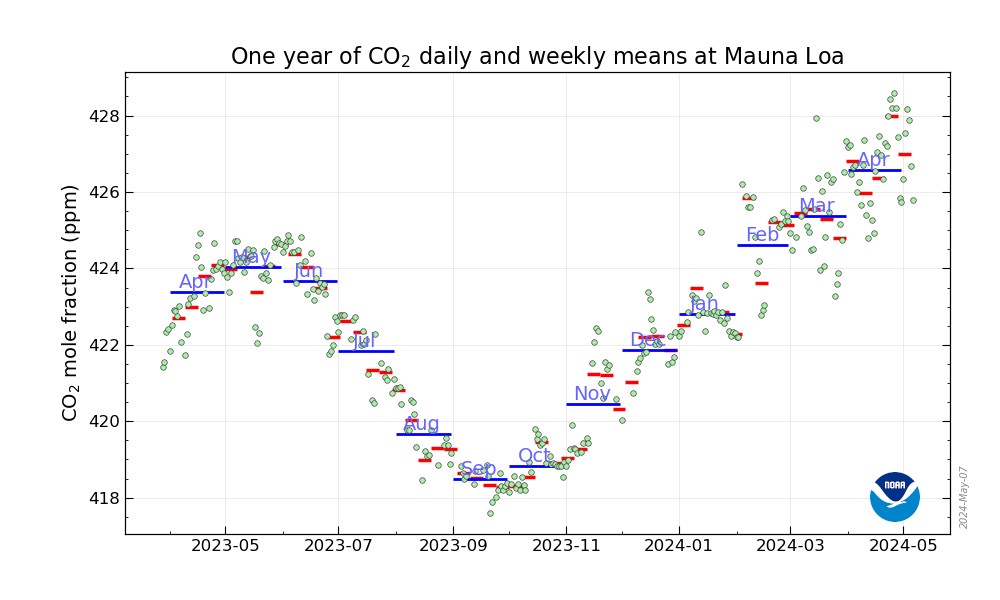
The weekly data page, the source of this graph, is here: Weekly average CO2 at Mauna Loa
Through most of 2020, the carbon dioxide concentrations have been running about 24.1 ppm over the figures for the same week of 2010.
I believe that ANY Supreme Court Justice who votes to dissolve the Constitution...
...should be impeached for perjury inasmuch as they have sworn to uphold it.
I just watched the 1993 political comedy "Dave" with Kevin Kline.
It's about a supremely corrupt President. An impersonator is convinced by a corrupt Chief of Staff to impersonate the President after he has a stroke while having sex with his secretary.
It's a pretty good movie, but the corrupt President was actually nowhere nearly as corrupt as that creature still in the White House but about to be escorted out ignominiously.
A funny part is when a conspiracy theorist actually is on television (on Larry King) and is actually right.
Conspiracy theorists, of course, have always had a focus on the Presidency, but no one imagined that an insane conspiracy President could actually be a really, really, really certifiable out of his gourd conspiracy theorist.
It was a simpler, more sane time, 1993.
The Glassy State of Matter.
(Note: This post, and many of my earlier posts in this space, contains some graphics which may not be accessible to Chrome users because of a recent upgrade to that browser, but should work in Firefox and Microsoft Edge. When my son has time, he will adjust the file system for a website he's building for me to make these graphics usable in Chrome, but he seldom has that much time on his hands. Interested parties, should they exist, can still read my posts including the graphics, but regrettably must use a browser other than Chrome. Apologies - NNadir)
The paper I'll discuss in this post is this one: The glassy state of matter: Its definition and ultimate fate (Edgar D. Zanotto, John C. Mauro Journal of Non-Crystalline Solids 471 (2017) 490–495) Dr. Mauro is at Corning. My son is finishing up his undergraduate education in Materials Science and will graduate this month formally and transition, at the same institution, into graduate school. He is already taking several graduate courses as he finishes his degree, one of which happens to be a course dedicated to glass.
Materials Science students in universities in this part of the country often apply for, and get, internships at Corning. My son, whose undergraduate research included work on certain kinds of non-crystalline ceramics, never applied to go to Corning for his internships, but his mother and I often discussed that it might be a good place for an internship.
Nevertheless, if one reflects on it, glass is a rather remarkable substance, the nature of which often confuses people.
When I was in high school, what seems like some centuries ago, my teacher - who inspired me to a career in chemistry by doing something no one would do today - exploding sodium in water, taught us that glass is a "liquid," that is, a fluid. He informed us that this surprising result could be realized by looking at glasses in very, very old houses, and noting that the glass was thicker at the bottom than the top, meaning it had flowed. (I never found glass old enough to actually observe this, but I believed him that such glass could be found, because I was, well, a kid.) For many years, I more or less believed this, even after completing my university education, at least until I was called upon professionally to consider glass transitions connected with certain classes of polymers, and until my son, who loves to talk down to me whenever he can as revenge for his upbringing, corrected me on this misapprehension.
The paper cited above, which is sort of geared for general audiences who are confused about what glass is and is not - a set of people that included my otherwise wonderful high school chemistry teacher (who is surely now dead of old age) - is designed to educate them accordingly. Here is the current title page, as of this writing, of the journal's latest collection of papers, the in progress issue: Journal of Non-Crystalline Solids, Volume 552 (in Progress) The word "glass" appears 30 times in the title page. Note that the journal is called Journal of Non-Crystalline Solids.
Glass is a solid, which seems fairly obvious on one level, although high school students like the one I used to be, are quite willing to believe it's a liquid when authoritatively told this is so.
This paper does a nice job explaining what the state of matter of glass is. The abstract, which should be accessible to the general public, includes a modern technical definition of what glass is, which for convenience, I quote here:
Here are two graphics from the paper displaying something about the nature of glass, the first of which shows the similarity to a supercooled liquid is not the same as the identity, because of the glass transition:

The caption:
The second graphic shows that a supercooled liquid need not pass through a glass state in order to crystallize:

The caption:
Note that neither graphic displays glass as being a supercooled liquid.
The paper reports some historical definitions of what a glass is:
In glass science and technology textbooks one finds several different definitions. In a historical monograph published in 1933, the Russian chemist Gustav Heinrich Johan Apollon Tammann [9] stated that “glasses are undercooled solidified melts.” Forty years later, Robert H. Doremus [10] pointed out that typical definitions of glass are represented by the following: “a material formed by cooling from the normal liquid state which has shown no discontinuous change in properties at any temperature, but has become more or less rigid through a progressive increase in its viscosity.” In 1976, J. Wong and C. Austen Angell [11] reported on the definition proposed by a committee of the US National Research Council: “Glass is an X-ray amorphous material that exhibits the glass transition. This being defined as that phenomenon in which a solid amorphous phase exhibits with changing temperature (heating) a more or less sudden change in its derivative thermodynamic properties such as heat capacity and expansion coefficient, from crystal-like to liquid-like values.” In that same year, Eduardo Mari [12] defined glasses as “amorphous solids that are obtained by fast cooling a molten mass averting its crystallization.” A few years later, in 1982, Jerzy Zarzycki [13] defined glass as “a non-crystalline solid that presents the phenomenon of glass transition.” In 2002, K. J. Rao [14] defined glass as “a solid obtained by supercooling a liquid and that is X-ray amorphous.” In the second edition of their book, in 2013, Ivan S. Gutzow and Juern W. Schmelzer [15] proposed a longer definition: “Glasses are thermodynamically non-equilibrium kinetically stabilized amorphous solids, in which the molecular disorder and the thermodynamic properties corresponding to the state of the respective under-cooled melt at a temperature T* are frozen-in. Hereby T* differs from the actual temperature T.” Finally, Arun K. Varshneya's [16], [17] definition is: “Glass is a solid having a non-crystalline structure, which continuously converts to a liquid upon heating.”
The key is the existence of a glass transition.
To be honest, I first learned about glass transitions when considering purchasing a differential scanning calorimeter to measure glass transitions in RNA, which is very unlike the stuff in a window or in a beer container, but that's where I first had to consider what a glass actually is. Here, from the text is technical definition of a glass transition:
More simply, a glass transition is determined by an inflection in a DSC curve, in which changes in temperature are plotted vs the heat flow within. The above definition sounds a little challenging, but in practice, finding the glass transition temperature is fairly straight forward.
The authors, in other definitions, make a distinction between an amorphous solid and a glass, both of which are non-crystalline solids, in as much as they lack periodicity.
The authors finally propose their own definitions of glass, one for the general public and one for technical use as follows:
An alternative, more elaborate description that clarifies the general properties of glasses is: “Glass is a nonequilibrium, non-crystalline condensed state of matter that exhibits a glass transition. The structure of glasses is similar to that of their parent supercooled liquids (SCL), and they spontaneously relax toward the SCL state. Their ultimate fate, in the limit of infinite time, is to crystallize.” This definition is for those who understand the meaning of glass transition.
They conclude as follows:
For many decades, researchers have attempted to define glass as either a liquid or, more typically, as a solid. However, this binary thinking does not do justice to the true complexity of the glassy state, which combines features of both liquids and solids and also brings along its own unique characteristics. Glass certainly appears to be solid on a typical observation time scale: it has mechanical rigidity and elasticity, and it can be scratched and even fractured, just as a solid. However, unlike a solid, a glass exhibits viscous flow and continuously relaxes toward the supercooled liquid state. This viscous flow behavior is more akin to that of the liquid state. In fact, the structure of a glass is similar to that of its corresponding supercooled liquid, which makes sense given that most glasses are formed by cooling from a melt. However, glasses also have properties that are unique to the glassy state due to their nonequilibrium and non-ergodic (frozen) nature. The properties of a glass depend not only on its composition and the current temperature and pressure, but also on the entire thermal and pressure histories experienced by the glass. Also, unlike either solids or liquids, glasses are thermodynamically unstable. Moreover, it has been previously shown that the glassy state itself cannot be expressed as any linear combination of supercooled liquid states [37], [38]. The glassy state is therefore truly unique and can be classified as its own (nonequilibrium) state of matter...
Glass is a meta-state, a thermodynamically unstable state that at equilibrium - which may take an infinite period of time - transition to a crystalline state.
I hope this discussion is useful.
Have a nice evening.
Norway's Prime Minister: Ocean science can boost jobs and wellbeing
This is a "World View" article in the current issue of Nature: Norway’s Prime Minister: Ocean science can boost jobs and wellbeing (Erna Solberg, Nature 588, 9 (2020))
An excerpt from the text:
I grew up on the west coast of Norway and my parents taught me how to fish when I was a little girl. I caught my first mackerel in a boat on the Hardangerfjord, and had it fried for dinner. Such memories become part of you. The ocean is central to Norway’s history and culture, economy and diet. We need it to weather existential threats — from the COVID-19 crisis to climate change. As the country’s prime minister, it is my job to ensure that our relationship with it is sustainable: protection, production and prosperity go hand in hand.
We cannot do this alone. In September, I went back to the west coast and spent time picking up plastic waste with volunteers. The plastic was from all over, much brought to Norway on ocean currents. Because the biggest threats to the ocean are now global, its safeguarding must be, too.
Perhaps the most notable international achievement to protect the ocean is the marine protected area around the Ross Sea in Antarctica. This 2016 agreement was hard won. More than two dozen nations agreed to preserve ecosystems in Antarctic waters, with 70% off-limits to fishing.
Protection is important to let damaged waters regenerate, but we need more: we can manage the ocean for its vast capacity to drive economic growth and equitable job creation, sustain healthy ecosystems, and mitigate climate change...
Really?
A good part of Norway's wealth comes from off shore oil and gas drilling in the North Sea.
Addressing that tiny matter would be a good start, no?
As I approach the end of my life, two things of which I am enormously proud is that...
...I never signed up for Twitter, and I never signed up for Facebook.
I may have lost face, but at least I can say I'm not a twit.
It made life worth living. ![]()
Even here on DU, there are more titles with "Trump" in them than those with "Biden."
This annoying habit is going on all over the media.
Trump is history, contemptuous history, but Biden is reality.
Personally I'm tired of what Trump has to say, what he does, what he thinks, what he tweets. It's time to ignore this sad diseased manchild.
This is Biden's time.
Let's forget diaper small hands.
Production of Thorium-229 in Oak Ridge Nuclear High Flux Isotope Nuclear Reactor.
(Note: This post, and many of my earlier posts in this space, contains some graphics which may not be accessible to Chrome users because of a recent upgrade to that browser, but should work in Firefox and Microsoft Edge. When my son has time, he will adjust the file system for a website he's building for me to make these graphics usable in Chrome, but he seldom has that much time on his hands. Interested parties, should they exist, can still read my posts including the graphics, but regrettably must use a browser other than Chrome. Apologies - NNadir)
The paper I'll discuss in this post is this one: Reactor production of Thorium-229 (Susan Hogle, Rose Ann Boll, Karen Murphy, David Denton, Allison Owens, Tamara J. Haverlock, Marc Garland, Saed Mirzadeh, Applied Radiation and Isotopes, Volume 114, 2016, Pages 19-27.)
I take much pride in my sons, but one of the proudest moments was when my youngest son was selected, after his sophomore year of college for a SULI internship at the Oak Ridge National Laboratory. It was, by all accounts, a remarkable experience for him. Besides the neutron spallation source at which he worked on his assignment, he got to tour many of the facilities there, including a tour of the High Flux Isotope Reactor, which, if I recall correctly, was not operating at the time because of a technical problem.
This reactor is one of the premier research and production tools in the United States, and represents a tremendous national resource.
It is the source of many important medical and research radioisotopes.
I was wandering in a desultory fashion through the literature concerning certain highly accurate timepieces and came across this interesting article about the production of thorium-229, an isotope under investigation for use in "nuclear clocks" which exceed the accuracy of "atomic clocks." This isotope does not occur naturally on earth, since the decay chain is the only one of the four actinide decay chains that has become extinct on Earth: It is a product of the decay chain of Californium-247/Curium-245/Neptunium-237 decay chain, which has no members - unlike the Thorium-232, Uranium-235, and Uranium-238 decay chains - with half-lives long enough to have survived the 4.5 billion years since the creation of the earth. Therefore all of the Thorium-229 on Earth is synthetic, and most of the world supply of it has surely been made at Oak Ridge.
This paper describes some aspects of scale up of this important isotope, focusing on its use in nuclear medicine.
From the introduction:
In this work, we report on a systematic evaluation of production of 229Th via neutron irradiation of 226Ra, 227Ac, and 228Ra targets in the ORNL High Flux Isotope Reactor (HFIR), examining the nuclear reactions involved in multiple production pathways and assessing the future capacity for reactor supply of 229Th. Some preliminary data from this research were presented earlier (Boll et al., 2005b).
Production of 229Th through neutron irradiation of 226Ra requires three neutron captures and two ?? decays through a number of pathways, shown in Fig. 1 along with their associated nuclear data.
Figure 1:

The caption:
Radium-228 does occur naturally on Earth, it is a member of the decay chain of thorium. However its half-life, 5.75 is very short in comparison to that of thorium-232, 14.1 billion years, with the result that its equilibrium concentration is extremely low, so low as to make its isolation from thorium ores impractical. Therefore radium-228 is also made synthetically, as described in the figure above, beginning with radium-226, which can be isolated from uranium ores, or uranium mine tailings, and, for that matter, from flowback water from fracking operations to obtain the dangerous natural gas that keeps the so called "renewable energy" fig leaf running.
Figure one shows that multiple different elements and isotopes are obtained in the neutron irradiation of radium-226, and the amount of each isotope present depends on the flux intensity, the half-lives of the elements, and their neutron capture cross-sections, a measure of how "large" they appear to a neutron, that is the probability that a neutron will strike a nucleus and be absorbed into it, producing an isotope that is one mass unit heavier, which may decay, via ?- decay, to an element adjacent to it with an atomic number 1 unit greater, as shown in the figure.
The quantity of each isotope of the involved elements is determined by the Bateman equation. From the text:

It is interesting to note what the flux in the "high flux" reactor is, and for positions in the reactor, this is shown in the following table:

This is a high flux indeed, about 3 orders of magnitude higher than what one might see in a commercial power reactor. The note about the "epithermal to thermal" issue is important, since the neutron capture cross sections are a function of the neutron speeds, which are equivalent to energy and are usually given in units of energy, eV, keV or MeV. A "thermal" neutron is one whose speed approximates the speed of a gas molecule at the ambient temperature, whereas "epithermal" are neutrons that have slowed from the very high (relativistic) speeds at which they are emitted, but not quite reached thermal speeds. "Fast" neutrons are neutrons that have not slowed appreciably from the speeds at which they are emitted, in fission, between 1-2 MeV.
The unit of cross section is a unit of area called "the barn," a whimsical name coined, for the purpose of secrecy, during the Manhattan Project from the idiomatic locution "couldn't hit the side of a barn." It approximates the area projected onto a surface by a three dimensional uranium atom; and is now formally defined as 1X10^(-28) square meters.
Here are the cross sections of the nuclei of interest in this system:

Here is the theoretical yield of the Th-229 isotope as it passes through irradiation cycles in the HFIR:

The caption:
The work involves multiple nuclei available from the inventory at ORNL:

A number of tables in the paper give yields of the various associated intermediate nuclei in comparison to the theoretical yields. I won't show those, but will show that for the target nucleus, thorium-229:

The thorium obtained is not monoisotopic thorium-229; it is mixed with other isotopes, including the very long lived natural isotope, 232, and an isotope occurring naturally in the U-238 decay series, Th-230, and the unusual isotope Th-228. This is shown in the following figure from the paper:

The caption:
The Th-228 isotope is very important in thorium based nuclear fuels owing to the Th-232(n,2n)Pa-231 side reaction giving rise to Th-231 which rapidly decays to Pa-231 in addition to the Th232(n,? )Th233 with Th233 decaying rapidly to fissionable U233. Neutron capture in Pa-231 yields the isotope U-232. U-232 has a half-life of 69 years, and all of its decay products are relatively short-lived. the longest lived being the aforementioned Th-228, with a half-life of only 1.9 years. The decay chain of this isotope is often evoked in nuclear weapons non-proliferation, since the decay series includes the isotope Tl-208, which is a very powerful gamma emitter, making the assembly of nuclear weapons nearly impossible, detection of them very easy, and their stability very poor.
In the present case, it's presence is not of much import, since the purpose of accumulating Th-229 is to milk it for its decay product, Ac-225, and the decay product of Ac-225, Bi-213 for radiotherapy. In any case the ratio of Th-228 to Th-229 will fall relatively rapidly, since Th-229 is fairly long lived, with a half-life of 7,917 years.
Th-228 isolated from decaying U-232 would make an excellent target for the production of additional Th-229.
The theoretical and actual yields of the isotopes in the tables excluded here are shown in this figure.

The caption:
The theoretical yield of Th-229 and Th-228:

The caption:
The ?-ray spectra of the irradiated and purified targets:

The caption:
There is a great deal of mysticism connected with nuclear technology, in which ignorance and fear substitute for knowledge. Nuclear power saves lives that would otherwise be lost to air pollution, and the restraint of the substitution of nuclear energy for dangerous fossil fuels actual kills people in vast numbers, unnecessarily and with malice, I might add.
However nuclear medicine also saves lives, and it is the work of people like the authors of this paper who make those life savings tools available. When I visited ORNL in connection with my son's internship there, I was moved to the core to reflect upon the great scientific and technological achievements that it made possible for the better of humanity. This is true even if it was initially constructed for warlike purposes, and in fact, still is engaged in weapons work. ORNL is a national scientific resource of immense value, and must be protected from fear and from ignorance in times where fear and ignorance are far too ascendent.
Have a pleasant and safe Sunday.
Proposed Constitutional Amendment 28.
"The right of the Senate to exercise advice and consent defined in Article II Section 2 to appointments by the President of the United States shall be limited to 180 days after the nomination shall have been transmitted to the Senate. If, after, a period of 180 days, the Senate has failed to act on the nomination, the President's nominee shall assume the office to which he or she has been nominated by the President."
I think this would have gone a long way to limiting the power of a thug like Moscow Mitch from working so hard to destroy the constitution.
Does anyone agree?
Indium Uptake and Accumulation by Rice and Wheat and the Health Risk Associated with Consumption.
(Note: This post, and many of my earlier posts in this space, contains some graphics which may not be accessible to Chrome users because of a recent upgrade to that browser, but should work in Firefox and Microsoft Edge. When my son has time, he will adjust the file system for a website he's building for me to make these graphics usable in Chrome, but he seldom has that much time on his hands. Interested parties, should they exist, can still read my posts including the graphics, but regrettably must use a browser other than Chrome. Apologies - NNadir)
The paper I'll discuss in this post is this one: Indium Uptake and Accumulation by Rice and Wheat and Health Risk Associated with Their Consumption (Hsin-Fang Chang, Puu-Tai Yang, Hui-Wen Lin, Kuo-Chen Yeh, Ming-Ni Chen, and Shan-Li Wang Environmental Science & Technology 2020 54 (23), 14946-14954)
Recently in this space, I discussed a paper on metal use and climate change in which I carried on about one of my favorite bugaboos, the question of whether so called "renewable energy" is actually "renewable" (or sustainable) given its dependence in some cases on relatively rare elements. One of my favorite bugaboo elements in these repetitive and annoying rants is the element indium, which I discussed at some length in that post, showing that an attempt to scale a particular type of thin film solar cell to anything approaching world energy demand, the CIGS (copper-Indium-Gallium-Selenide) cell would easily deplete all the known reserves of that element. That post is here: Material Flow Analysis (MFA): Metal Demand and Climate Change.
Despite approximately half a century of cheering for it, and the expenditure of trillion dollar quantities of resources on it, the solar industry remains a trivial form of energy that has consistently failed to address climate change, and to be fair, the CIGS portion of the trivial but much worshipped solar industry is trivial therein.
The expansion of the isolation of indium from zinc mining activities is largely due to the importance of this element in touch screen electronic devices, notably cell phones and computer monitors, where it is the form of a tough, electrically conducting transparent metalloceramic, indium tin oxide.
The occupational risk associated with indium workers is well known, and is known as "indium lung" which in its effects, as I noted in that previous post, very much like black lung disease among coal miners. There are a significant number of publications on this topic, mostly in occupational health journals, but an interesting mechanistic paper appears in the open sourced journal of the Nature family, Scientific Reports: Nitrative DNA damage in lung epithelial cells exposed to indium nanoparticles and indium ions (Ahmed, S., Kobayashi, H., Afroz, T. et al. Nitrative DNA damage in lung epithelial cells exposed to indium nanoparticles and indium ions. Sci Rep 10, 10741 (2020).)
While indium lung is associated with breathing indium tin oxide dusts in electronics manufacturing (or recycling) plants, the mechanism proposed, DNA damage, suggests that it may not be any better an idea to eat indium than it is to breathe it.
The paper cited at the outset thus discusses the biochemical transport of indium into food, notably rice and wheat. From the introduction to the paper:
According to various surveys reported in the literature, plants can absorb indium found in surrounding soils.(8?12) In soils containing 0.15–0.41 mg kg–1 indium, the accumulation of indium was up to 5 ?g kg–1 in wheat and barley, and up to 50 ?g kg–1 in perennial ryegrass.(8,9) Cabbage, garlic, and water spinach were found to accumulate 2–5 ?g kg–1 indium from soil containing 1.38 mg kg–1 indium.(10) Relatively high concentrations (8–2100 ?g kg–1) accumulated in beets(11) and grass(12) collected near indium-associated industrial areas, with soils containing 0.5–4.2 mg kg–1 of indium. Indium accumulation in plants can hinder the uptake of mineral nutrients given that indium does not have a specific transporter.(13) Alternatively, the accumulation itself may adversely impact plant growth and development.(13?15)
Rice (Oryza sativa L.) and wheat (Triticum aestivum L.) are two predominant global cereal crops. Due to the high dairy consumption rates of rice and wheat grain by humans, the bioaccumulation of any contaminants in them can pose a higher risk to human health than those in other crops. However, the effect of cultivating these crops in indium-contaminated farmland on human exposure has not been well understood. Therefore, this study aims to investigate the uptake and accumulation of indium by rice and wheat and to assess the risk associated with grain consumption...
Here's the cartoon associated with the paper:

The authors grew these plants in soils amended deliberately with indium at concentrations known in fields close to electronics plants in Taiwan:
They then used several instrumental methods, ICP-OES, Laser ablation ICP/MS (LAICP/MS), XAS, etc to track how indium traveled in food plants. (I personally prefer ICP/MS to ICP-OES because of its greater sensitivity and wider dynamic range, but OES works for this purpose.)
Some pictures from the text:

The caption:
These letters are explained in the text with respect to the origin of the soils tested:
Some text is useful to grasp the meaning of figure 2:
Table 1:


The caption:
To obtain the form of the indium in the soils, a sequential extraction procedure known as BCR was utilized:

The caption:
The plants were allowed to grow until they yielded grain, and then analyzed for indium content:

The caption:
Indium is one of three elements - the other two are tellurium and rhenium - in which the radioactive (unstable) isotope is present to a larger extent than the stable isotope(s). Indium 115 is radioactive, but its very long half-life means its radiological risk is trivial. However, the very long half-life of cadmium-113, which decays to indium's only stable isotope, In-113, has prevented much indium-113 from accumulating, since the half-life of cadmium-113 is much longer than the age of the earth. For the purpose of mass spectrometry therefore, the common (radioactive) isotope In-115 was chosen as shown in the following image:

The caption:
Table 2, bioaccumulation factors:

Daily intake figures:

These figures are lower than reference levels of concern, but over a period of time, depending on biological half-life, they may have health implications. These may be exacerbated by using the grains as animal feed, where bioaccumulation in food animals can result in troublesome exposure.
From the conclusion:
Other indium exposure pathways must be identified to fully assess its risk to human health. As mentioned in the Introduction, vegetables like cabbage, garlic, and water spinach can take up 2–5 ?g kg–1 indium from soil containing 1.38 mg kg–1 indium.(10) Their corresponding BAF values are from 1.4 × 10–3 to 3.6 × 10–3, which is similar to the BAF values of rice and wheat shoots (Table 2). Because BAFr and BAFs values are generally higher than BAFg values, and because people consume greater biomass when eating vegetables, the consumption of leafy greens and root vegetables may still pose a health risk in terms of indium exposure. However, it is important to note that dietary practices vary across the globe, therefore complicating HQ calculation. Further studies on indium residence time in soil and plant systems, as well as the human body, would be highly informative and important, given that very few studies have been conducted. Moreover, the straws of rice and wheat may be used as feedstock for farm animals, which has the potential for being a large pathway for indium assimilation into the human body. Dust inhalation and dermal contact are also suggested as possible sources of indium bioassimilation(41), because human daily soil ingestion rates range from 37 to 625 mg of soil.(42) Ingestion of indium-containing soil by farm animals, such as geese (soil ingestion is 8.2% of their diet), sheep (9.5% of diet), and cattle (9.0% of diet),(43) may contribute to human indium exposure. Information regarding the contribution of various indium exposure pathways to humans is essential for defining the safe limit allowed in agricultural soils.
It's something to keep our eyes on, but for now, indium is mostly toxic in occupational settings.
Have a pleasant, and above all, safe weekend.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,509