Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Environment & Energy
Related: About this forumThe KrCl* excimer lamp for UV irradiation a 222 nm.
I am always interested in the use of radiation to destroy water contaminants.
I came across this paper this evening: UV 222 nm Emission from KrCl* Excimer Lamps Greatly Improves Advanced Oxidation Performance in Water Treatment Emma M. Payne, Bryan Liu, Lauren Mullen, and Karl G. Linden Environmental Science & Technology Letters 2022 9 (9), 779-785.
I don't have much time to fully discuss this paper, but I thought it worth excerpting briefly and posting a figure from it.
222 nm is a shorter wavelength (higher energy) radiation than traditional mercury UV lamps.
An excerpt:
Ultraviolet (UV)-based advanced oxidation processes (UV/AOPs) are frequently employed in municipal drinking water and water reuse treatment for micropollutant abatement. Common UV/AOPs utilize hydrogen peroxide (H2O2) to generate hydroxyl radicals (•OH) under monochromatic low-pressure mercury vapor lamp (LPUV) irradiation at 254 nm or broad spectrum (200–800 nm) irradiation from medium-pressure mercury vapor lamps. This UV/AOP generates hydroxyl radicals via hydrogenperoxide photolysis, which react rapidly (rate constants of 10^07–10^10 M^(–1) s^(–1)) and nonselectively with many organic and inorganic contaminants. (1,2) In recent years, the UV-driven production of hydroxyl radicals from species other than hydrogen peroxide has generated interest. AOPs that use chlorine (UV/Cl2 or UV/NH2Cl) (3?5) or peroxydisulfate (UV/PDS) (6,7) as the radical promoter are increasingly being studied and, in some cases, employed at pilot or full scale. (8?11) Additionally, water matrix constituents such as nitrate or iron can create de facto AOPs when exposed to UV light. (12?15) Nitrate absorbs light very strongly at wavelengths below 240 nm to produce hydroxyl and other radicals and has demonstrated potential as a radical promoter in UV/AOP systems that utilize light sources emitting at far-UVC (200–230 nm) wavelengths. (16?18)
Coinciding with the investigation of alternative radical promoters are advancements in UV source technology. Both germicidal UV light-emitting diodes and excimer (or exciplex) lamps have emerged in the past several decades as potential tools in UV-based water treatment technology. (19?21) Of particular interest here are excimers, which generate deep UV emission when a rare gas or rare gas–halogen dimer returns to the ground state from its excited state, with the emission wavelength corresponding to the composition of the dimer. (22,23) KrCl* excimers emit narrow-band UV light at 222 nm and have recently been studied for use in disinfection, (20,24,25) greatly enhancing the disinfection of UV-resistant viruses, (26) including for control of SARS-CoV-2. (27?29) KrCl* excimers have advantages over conventional UV sources because they do not contain mercury and the far-UVC emission at 222 nm is not very harmful to human tissue, (30?32) thereby eliminating several hazards typically associated with conventional UV treatment of surfaces, air, and water. Few studies have exploited the unique wavelength emission of KrCl* excimers for improving advanced oxidation. (33?36)
The goal of this study was to promote radical improvements in AOP treatment through the use of 222 nm emitting lamps by comparing •OH generation, as measured by a probe compound, across three UV advanced oxidation systems: conventional LPUV with hydrogen peroxide as the radical promoter (LPUV/H2O2), KrCl* excimer with hydrogen peroxide (KrCl*/H2O2), and KrCl* excimer with nitrate (KrCl*/NO3–). Experiments were conducted in both ultrapure water and a natural groundwater to investigate the rate of •OH production and the impacts of background water constituents on the 222 nm-driven generation of •OH in comparison to conventional LPUV emitting at 254 nm. The opportunities for process optimization by utilizing new UV sources or radical promoters are presented, and the challenges of quantitatively comparing different UV/AOPs are described. Lastly, areas of needed research are proposed to support adoption of 222 nm-driven AOPs...
Coinciding with the investigation of alternative radical promoters are advancements in UV source technology. Both germicidal UV light-emitting diodes and excimer (or exciplex) lamps have emerged in the past several decades as potential tools in UV-based water treatment technology. (19?21) Of particular interest here are excimers, which generate deep UV emission when a rare gas or rare gas–halogen dimer returns to the ground state from its excited state, with the emission wavelength corresponding to the composition of the dimer. (22,23) KrCl* excimers emit narrow-band UV light at 222 nm and have recently been studied for use in disinfection, (20,24,25) greatly enhancing the disinfection of UV-resistant viruses, (26) including for control of SARS-CoV-2. (27?29) KrCl* excimers have advantages over conventional UV sources because they do not contain mercury and the far-UVC emission at 222 nm is not very harmful to human tissue, (30?32) thereby eliminating several hazards typically associated with conventional UV treatment of surfaces, air, and water. Few studies have exploited the unique wavelength emission of KrCl* excimers for improving advanced oxidation. (33?36)
The goal of this study was to promote radical improvements in AOP treatment through the use of 222 nm emitting lamps by comparing •OH generation, as measured by a probe compound, across three UV advanced oxidation systems: conventional LPUV with hydrogen peroxide as the radical promoter (LPUV/H2O2), KrCl* excimer with hydrogen peroxide (KrCl*/H2O2), and KrCl* excimer with nitrate (KrCl*/NO3–). Experiments were conducted in both ultrapure water and a natural groundwater to investigate the rate of •OH production and the impacts of background water constituents on the 222 nm-driven generation of •OH in comparison to conventional LPUV emitting at 254 nm. The opportunities for process optimization by utilizing new UV sources or radical promoters are presented, and the challenges of quantitatively comparing different UV/AOPs are described. Lastly, areas of needed research are proposed to support adoption of 222 nm-driven AOPs...
A figure from the paper:
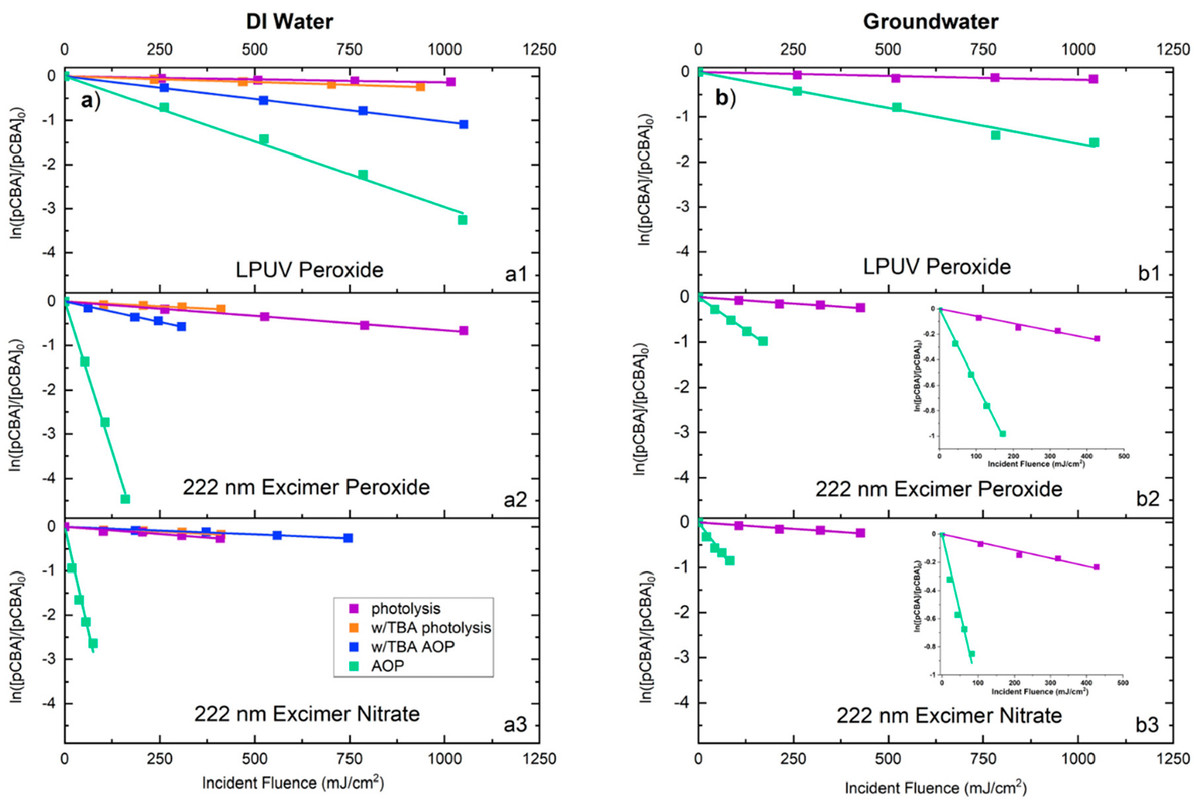
The caption:
Figure 1. (a) pCBA degradation in three UV/AOPs in ultrapure deionized water. (b) pCBA degradation in three UV/AOPs in groundwater. The slopes of all linear regressions (i.e., rate of pCBA degradation) are presented in Tables S3 and S4.
"pCBA" is parachlorobenzoic acid, a model aryl chlorocompound for far more toxic pollutants, for example, PCBs, polychlorobiphenyls, which represent a major pollution problem.
The krypton halide source suggests to me a use for the fission product Kr-85, an isotope produced in about 0.7% of fast fission of plutonium, which has a half-life of 10.576 years. Presumably this isotope can be recovered from liquid nuclear fuels (or fresh solid nuclear fuels) in order to generate this kind of UV source for the continuous generation of 222 nm UV light without requiring a power source.
Cool, I think.
InfoView thread info, including edit history
TrashPut this thread in your Trash Can (My DU » Trash Can)
BookmarkAdd this thread to your Bookmarks (My DU » Bookmarks)
0 replies, 830 views
ShareGet links to this post and/or share on social media
AlertAlert this post for a rule violation
PowersThere are no powers you can use on this post
EditCannot edit other people's posts
ReplyReply to this post
EditCannot edit other people's posts
Rec (3)
ReplyReply to this post