Environment & Energy
Related: About this forumSeriously Psychedelic Photograph Of Methane Bubbles Migrating Upward In Arctic Ice - NPR

EDIT
They are gas bubbles, little hiccups of methane that look magical when they're trapped in winter ice, but come the spring, those bubbles will loosen, get free, and like an armada of deep-water flying saucers, they will make their way to the surface. When the ice breaks they will pop and fizz into the air — and disappear. Except they don't really disappear. Once they hit air, methane bubbles make trouble. How much trouble depends on how many bubbles get released all over the planet. In this one lake, there are thousands, tens of thousands of them, as you can see. But in the oceans, they are bigger — much bigger.
Methane gas comes from leaves (and trees and grass even dead animals) dropping into the water, where they sink to the bottom and get munched on by bacteria that poop out methane, producing that familiar "marsh gas" smell. Some gas is much older, squeezed out of ancient oceans or from deep down near the Earth's mantle. When that older methane rises to the surface and bumps into freezing lake or ocean water, it fuses into a hard white substance called methane hydrate, a white, pasty rock. As long as it's frozen at the lake bottom, the gas is trapped, but when it warms, the gas fizzles out of the rock or mud, forming these lava lamp clumps that float up in six, seven, ten foot columns, like this ...

EDIT
Look at any one of these flat little lake-trapped pancakes, and now imagine one 3,000 feet wide — more than a half a mile across. Gargantuan methane bubbles do exist. Not in lakes, but in the Arctic Ocean. No one had ever seen them that big, or measured them until a couple of summers ago a Russian researcher Igor Semiletov and his wife Nadia, working with an American team, found more than a hundred of them in a small section of the arctic sea off Siberia.
"These are methane fields on a scale not seen before," he reported. "In a very small area, less than 10,000 square miles, we have counted more than 100 fountains, or torch-like structures, bubbling through the water column." he said.
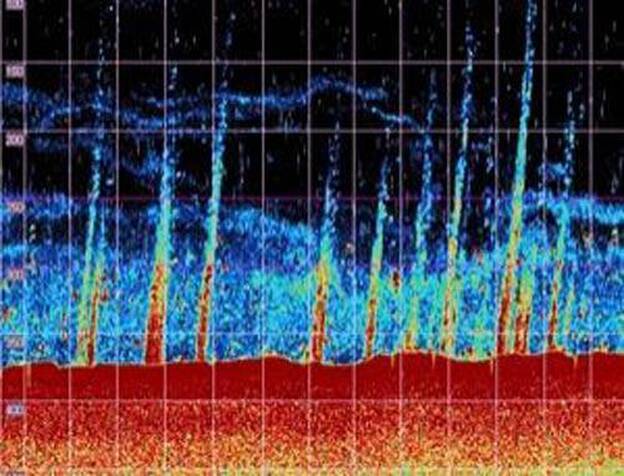
EDIT
http://www.npr.org/blogs/krulwich/2013/01/30/170661670/pale-blue-blobs-invade-freeze-then-vanish
GeorgeGist
(25,322 posts)MuseRider
(34,115 posts)I was trying to think of something like that. Good one.
Beautiful but deadly.
phantom power
(25,966 posts)
hatrack
(59,592 posts). . . . but nicely played! ![]()
phantom power
(25,966 posts)or, tales from canfield we're-fucked oceans?
I like this game.
hatrack
(59,592 posts)"Fragile" was a bit obvious, I thought.
Hissyspit
(45,788 posts)
yodermon
(6,143 posts)alterfurz
(2,474 posts)...that the light from fucked won't reach us for 10,000 years. -- Roseanne Barr

UnrepentantLiberal
(11,700 posts)Did Methane Cause the Mass Extinction That Made Way for the Dinosaurs?
By Veronique Greenwood
Discover
July 25, 2011
Two hundred million years ago, half of the Earth’s species vanished in the blink of a geological eye, clearing the way for rise of the dinosaurs in the Jurassic. The cause of that mass extinction, a new study suggests, may have been gigatons of methane released from the sea floor after a slight rise in the earth’s temperature, triggering much greater warming. And if that sounds familiar, it’s because scientists are worried the same thing will happen today.
The primary theory as to what went wrong at the end of the Triassic period, when this extinction took place, holds that tons of carbon dioxide released during the breakup of the supercontinent Pangaea ratcheted up global temperatures to deadly levels over the course of several hundreds of thousands of years.
But these researchers’ work seems to indicate that the change took place even more quickly than that. In a previous study looking at limestone, which is the remains of ancient sea creatures, this team found that it disappeared from the geological record quite suddenly—a mere 20,000 years after the extinction event began.
For this study, they turned their attention to the cause of that extinction, looking into whether higher levels of methane could have been behind the warming event.
More: http://blogs.discovermagazine.com/80beats/2011/07/25/did-methane-cause-the-mass-extinction-that-made-way-for-the-dinosaurs/#.USTnpZPCv5Y
wtmusic
(39,166 posts)caseymoz
(5,763 posts). . . is it possible to ignite these? Or even capture them? I mean methane, that's what we call "natural gas."
I admit that will release CO2, but that's not as bad a greenhouse gas as methane, which is supposed to be, what, more than an order of magnitude worse?
This is what I don't get about the chemistry: methane might be a much worse greenhouse gas than CO2, but it's also very reactive. CO2 stays around forever, but there's no way methane could last in the atmosphere very long. It has to oxidize.
I don't mean to sound like a denier. I'm just looking for loopholes.
mopinko
(70,178 posts)you would think that at least the frozen hydrates could be collected.
i do think we are fucked, tho.
CRH
(1,553 posts)is more CO2. Actually the chemistry is fairly straight forward. In the atmosphere methane reacts several ways in different feedback loops, when it is converted. So when it is gone, it really isn't gone, the effects linger.
It is thought to be about twenty five times as potent a green house gas as CO2.
Conversion time depends where in the atmosphere it is located, with the average time being 8.4 yrs. The bi-products of conversion are generally water vapor and CO2. Water vapor is another GHG and is yet another positive feedback cycle.
Here is a good primer -
http://en.wikipedia.org/wiki/Atmospheric_methane
Reaction with the hydroxyl radical- The major removal mechanism of methane from the atmosphere involves radical chemistry; it reacts with the hydroxyl radical (·OH) in the troposphere or stratosphere to create the CH·3 radical and water vapor. In addition to being the largest known sink for atmospheric methane, this reaction is one of the most important sources of water vapor in the upper atmosphere.
CH4 + ·OH ? ·CH3 + H2O
This reaction in the troposphere gives a methane lifetime of 9.6 years. Two more minor sinks are soil sinks (160 year lifetime) and stratospheric loss by reaction with ·OH, ·Cl and ·O1D in the stratosphere (120 year lifetime), giving a net lifetime of 8.4 years.[1] Oxidation of methane is the main source of water vapor in the upper stratosphere (beginning at pressure levels around 10 kPa).
~~ again
Troposphere
The most effective sink of atmospheric methane is the hydroxyl radical in the troposphere, or the lowest portion of Earth’s atmosphere. As methane rises into the air, it reacts with the hydroxyl radical to create water vapor and carbon dioxide. The lifespan of methane in the atmosphere was estimated at 9.6 years as of 2001; however, increasing emissions of methane over time reduce the concentration of the hydroxyl radical in the atmosphere.[11] With less OH˚ to react with, the lifespan of methane could also increase, resulting in greater concentrations of atmospheric methane.
Stratosphere
Even if it is not destroyed in the troposphere, methane can usually only last 12 years before it is eventually destroyed in Earth’s next atmospheric layer: the stratosphere. Destruction in the stratosphere occurs the same way that it does in the troposphere: methane is oxidized to produce carbon dioxide and water vapor.
end edit
Water Vapor as a GHG and feedback can be found here -
http://www.science20.com/news_account/greenhouse_gases_and_water_vapor_when_positive_feedback_is_a_bad_thing
~~ The water vapor feedback mechanism works in the following way: as the atmosphere warms due to human-caused increases in carbon dioxide, methane, nitrous oxide, and chlorofluorocarbons, water vapor increases, trapping more heat in the atmosphere, which in turn causes a further increase in water vapor.
Basic theory, observations and climate model results all show that the increase in water vapor is roughly 6 percent to 7.5 percent per degree Celsius warming of the lower atmosphere.
end
caseymoz
(5,763 posts)At 9.6 years, that's still pretty short (longer than I would have thought, though). CO2 and water vapor are extremely stable and could stay around a much longer time.
If it's worse than CO2 by 25 fold, then burning a molecule of methane yields only two water molecules and one CO2 molecule. Water, I think, is comparable to CO2 as a greenhouse gas.
Therefore, it seems to me by burning it, you have 1/8th the problem, in the short-run. In the long run, the CO2 and water vapor will hang around forever, but eventually, it seems the methane will break down into those two components anyway. So the shortcut might be better.
Viking12
(6,012 posts)MjolnirTime
(1,800 posts)AtheistCrusader
(33,982 posts)progressoid
(49,992 posts)to do that to all the lakes and marshes in Alaska, Canada, Russia, etc is impractical. Impossible even.
Bernardo de La Paz
(49,027 posts)GliderGuider
(21,088 posts)Energy-seeking behavior is the fundamental, universal characteristic of all living organisms. We inherited that requirement from the beginning of life on the planet. Because it is so crucial to survival, this behavior is encoded as the base characteristic of our genome, and so it ultimately manifests in every action, social structure and institution we create. Because it's buried so deep in our structure (as it is with all life), it's virtually impossible to recognize, let alone to recognize it as a threat and try to resist it.
When teamed with the problem-solving skills of our brain, this energy-seeking behavior has taken us from foraging to agriculture to industry, from wood-burning to coal to nuclear power without a murmur. Any cost was acceptable in order to get more energy. The only time we tried to fight against the energy-seeking instinct was when the symptoms became universally life-threatening.
Even now we only fight piecemeal, against easy targets like nuclear power. The biggest threats still pass unchallenged - we would much rather fight "global warming" than our own energy-seeking behavior. Unfortunately, the time lag inherent in the end-product of CO2 has made even that partial recognition come between 50 and 200 years too late.
Hissyspit
(45,788 posts)wtmusic
(39,166 posts)"Leave it to the Canadians to light Nature's farts on fire."
![]()