Science
Related: About this forumMore of the same: An exciting boost for solar cells!!!!!!!!
The paper I'll discuss in this post is this one: Sensitization of silicon by singlet exciton fission in tetracene (Baldo et al, Nature. 571, 90–94 (2019). The paper is probably not open sourced but the news item discussing it may be: An exciting boost for solar cells. I took the title of this post from the news item adding my own editorial comment to it: I have convinced myself that so called "renewable energy," as popularly imagined, is useless as strategy to address climate change, even though the delusional faith - and inasmuch as it is delusional it is also pernicious - persists that it will do so. It has not done so. It is not doing so. It won't do so.
Of course, as a political liberal, I was certainly not immune to believing it would do so, and years ago I certainly would have applauded the idea of spending trillions on it, which we have done, but the result of this experiment is written in the planetary atmosphere:
Up-to-date weekly average CO2 at Mauna Loa
Week beginning on June 23, 2019: 413.35 ppm
Weekly value from 1 year ago: 410.73 ppm
Weekly value from 10 years ago: 388.54 ppm
Last updated: July 4, 2019
There is, as many of us know, but certainly not all of us, a big, big, big difference between belief and fact.
In science, if there is a theory that disagrees with the experimental result, the theory goes, not the experimental result. Again, the measurement of the experimental result is in, as measured above at Mauna Loa.
My whole adult life - and I'm not young - I've been reading all about "breakthroughs" in so called "renewable energy," having read many of them in the E&E forum right here. I'm sure if you mosey over there, you can still read them.
The news item includes text that features the bad thinking that has gotten us into this nearly or totally intractable mess of climate change. It is these sentences:
The first sentence is, by the way, true. All agricultural energy - including human and animal muscle power - derives from the sun. This should inspire some critical thinking, but generally in popular discourse doesn't. There is a reason that humanity abandoned "renewable energy" in the 19th century, as I often state. The reason is that most people lived short, miserable lives of dire poverty. In fact, if one cares to look, this is still a factor: Slightly less than half of the 7 million people who die each year from air pollution are in fact killed by the waste products of biomass combustion (cigarettes not included here) and overwhelmingly these are poor people who live much as people might have lived hundreds of years ago.
The second sentence, the one including that magical word so badly abused by advocates of so called "renewable energy," "watts" is rather disgusting, since it implies that there is plenty of energy from the sun and the "only" thing that we need to do is to collect it. This is garbage thinking, although advocates of what I personally regard as the only environmentally acceptable and sustainable form of energy, nuclear energy, were quite nearly destroyed by the application of such thinking.
To wit:
A kg of uranium, when transmuted into plutonium, contains about 80.2 trillion Joules of energy. This is roughly the energy equivalent of 620,000 gallons of gasoline. The world energy demand as of 2017, as reported by the international energy agency, was 584 exajoules, which corresponds to average continuous power of 18.5 trillion watts. Thus all of the world's energy demand could be met by fissioning about 230 grams of plutonium each second.
When I was a kid, pre-adolescent, my mother, a completely uneducated woman who was nonetheless interested in my education, bought me a "toy" that one almost certainly not buy today, a small eye piece that if I held it to my eye in a closet allowed me to "see" radioactivity. I spent hours with that thing and I recall that the description from the notes that came with the thing of the flashes that I saw while sitting in a dark closet, fascinated, said that I was seeing individual atoms decay.
It was probably true. I suspect that the toy consisted of a phosphorescent screen impregnated with radium (which I had on my boyhood clock in much larger amounts) or polonium or some such isotope. The toy lead me to believe that "the atom" contained enormous amounts of energy, which of course it does.
However, as we all know, and as we are often reminded by anti-nuke appeals to stupidity and ignorance, it is not the energy content of plutonium that matters; what matters is the cost - and I would argue - the environmental impact of the device for collecting the energy that matters. Even though the United States built more than 100 nuclear reactors in the period between 1957 and 1980, using technology developed in the 1950's and 1960s, while providing some of the cheapest electricity on Earth, this while saving human lives that would have otherwise been lost to air pollution, we now hear that nuclear energy is "too expensive." In other words, what has already happened is now considered impossible.
Well then...
The same that applies to the nuclear industry - the cost of the device as opposed to the total quantity of energy - applies to the solar industry, it is the device that matters, not the energy flux of solar energy, and as the device matters, so does its environmental impact, which is largely ignored whenever the issue of so called "renewable energy" is discussed in a rote - but in my view nonsensical - evocation in which it is routinely described as "green." The word "green" of course, has come to mean "sustainable" and "environmentally benign" which the solar industry is decidedly not. Since the solar industry cannot produce continuous energy - it is widely reported and generally accepted that the sun goes "down" every evening - any discussion of its cost in terms of its internal - dollars per unit of energy paid by the consumer - costs and its external costs - the environmental destruction its use incurs and which is charged to all current and future generations - should accrue to solar energy, but since, as we are accustomed with and happy to lie to ourselves, is not. A battery is an energy wasting device, per the second law of thermodynamics, and overall batteries are not sustainable devices since their environmental cost is not acceptable or sustainable.
So this brings me to the paper under discussion, the solar "breakthrough" du jour.
From the news item:
The first sentence, as anyone who has lived as long as I have will have listened to half a century of cheering like madmen and madwomen for solar energy, should give some pause for critical thinking, but it won't.
The rest of this excerpt is nonsense, except for the fact that "...researchers are continually driven to make these devices..." which is largely a function of grant money, a cultural rather than technological imperative. (Scientists are not immune from culture; the are in fact a part of culture and what they do is a function of culture.)
Now let's turn to the paper itself, which has some very interesting science, even though the application of this science is not what it would seem from the description in the news item.
From the abstract, as Nature often uses abstracts as part of the content:
Sounds great!!!
From the main text:
As I noted recently in a recent post here in connection with the "miracle" cesium lead iodide perovskite solar cells, lead, a toxic element in most places becomes instantly "green" when attached to the word "solar."
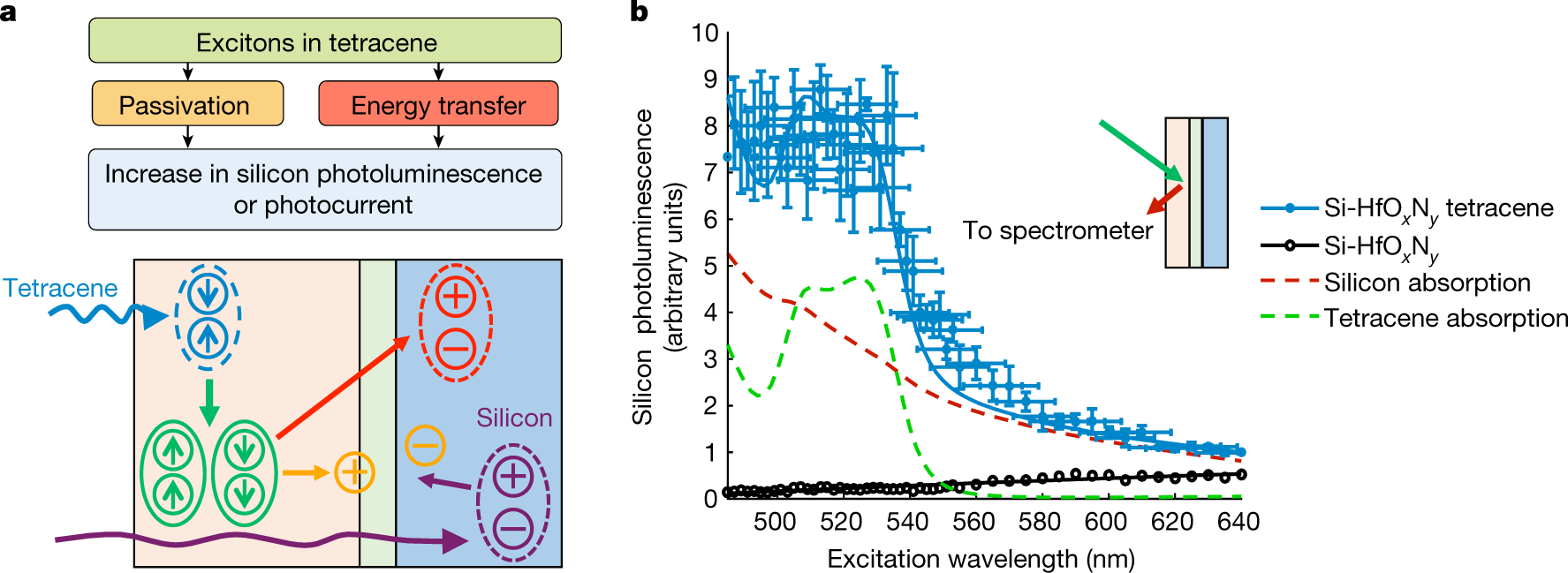
Figure 1:

The caption:
To continue with the text:
The authors discuss the need for precise control of the thickness of the passivation layer, essentially the construction of a layer on an atomic scale. This is because the transfer of electrons depends on quantum mechanical tunneling, which decreases with the thickness of the layer through which it must "tunnel." The interesting approach involves the remarkable advances in nanotechnology, in this case, atomic layer deposition. For this purpose they chose to utilize hafnium oxynitride to make this layer since its deposition properties on silicon is well understood and controllable.
Although solar energy is magically green, the scientist in me, the skeptic on how "green" all of this really is was inclined to view the processing described herein:
For a schematic of the fabrication process, see Extended Data Fig. 2. We purchased silicon wafers from PureWafer (for details see Supplementary Table 3) and transferred them to a clean room. We then performed a standard Radio Corporation of America (RCA) clean as follows. First, organic contaminants were removed by immersing the sample in a 5:1:1 ratio solution of deionized water, aqueous ammonium hydroxide (29%), and aqueous hydrogen peroxide (30%) for 20 min at 80?°C. Second, the native oxide was removed by etching with aqueous hydrofluoric acid (1%) for 60 s. Third, metal-ion contaminants were removed from the wafer surface in a 5:1:1 ratio solution of deionized water, aqueous hydrochloric acid (37%), and aqueous hydrogen peroxide (30%) for 20 min at 80?°C. We then deposited 10 nm of aluminium oxide on both sides using atomic layer deposition. Using photolithography, we fabricated electron-selective contacts (aluminium/lithium fluoride) and hole-selective contacts (aluminium/molybdenum oxide) in an interdigitated fashion. We then diced the wafers into chips and protected the back side of the individual chips using PDMS and a second piece of encapsulating silicon to provide a seal. We experimentally verify in Extended Data Fig. 10 that a full RCA clean before HfOxNy deposition is not compatible with our solar cell fabrication because the protective rear PDMS seal decomposes in the presence of acids, and the monomers subsequently reattach to the front side by condensation27,28. Consequently, to minimize contamination by PDMS in this process, we cleaned the front side using 10% aqueous hydrofluoric acid for 1 min and a 5:1:1 ratio solution of deionized water, aqueous ammonium hydroxide (29%) and aqueous hydrogen peroxide (30%) for 20 min at 60?°C. We fabricated the downconversion front side as detailed below and then peeled off the silicon protecting the back contacts (as shown in Extended Data Fig. 2).
PDMS is polydimethylsiloxane. It is made by passing chloromethane over silicon, the silicon having been produced by reduction with carbon.
If one is an environmentalist, in my opinion, as opposed to a person engaged in mindless hand-waving and wishful thinking, it useful to look at these lab scale processes and to consider what might be involved in scaling them up to a scale that mattered. It is also useful to consider that right now, in 2019, with concentrations of the dangerous fossil fuel waste carbon dioxide having reached 415 ppm this year in the atmosphere, the entire so called "renewable energy" industry is trivial. Combined, solar and wind and geothermal and tidal energy produced, as of 2017 less than 11 exajoules of the 584 exajoules humanity generated and consumed that year.
Anyway the hafnium oxynitride layer on silicon works great! (In the lab...)
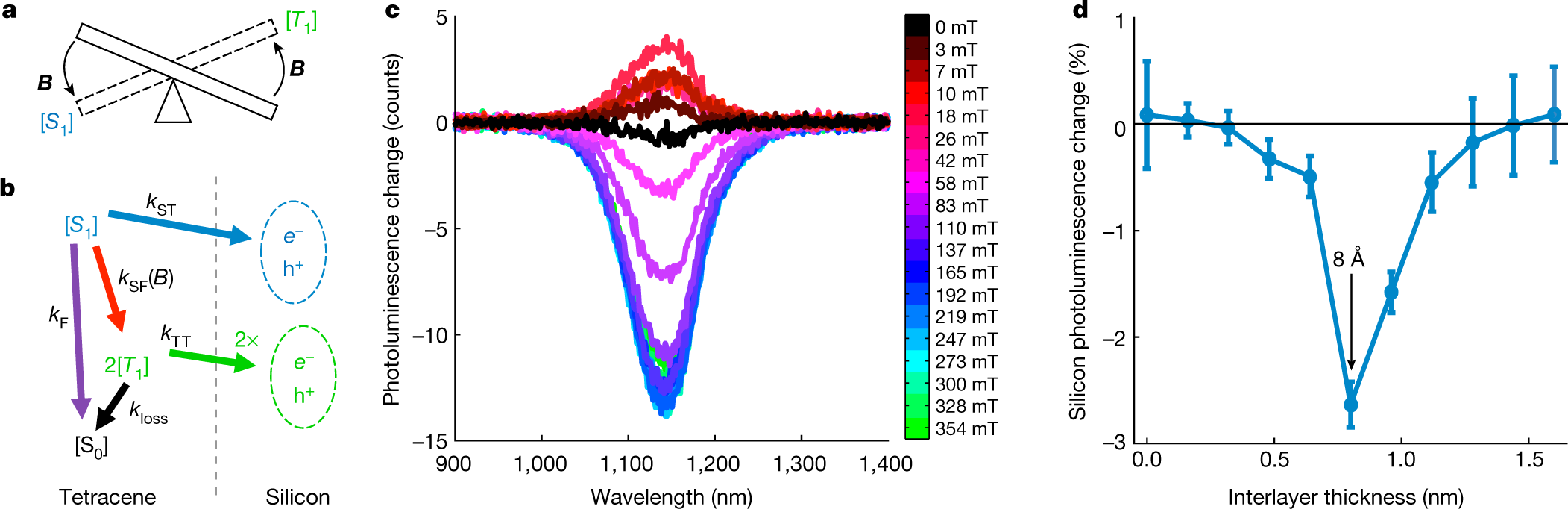
Some pictures from the paper:

The caption:
Another graphic:
The caption:
Simplistic..simplistic...
We have simply bet the future of humanity - of all future generations - on the ability of so called "renewable energy" to save the day. Are we tired of all this "winning?"
Anyway:
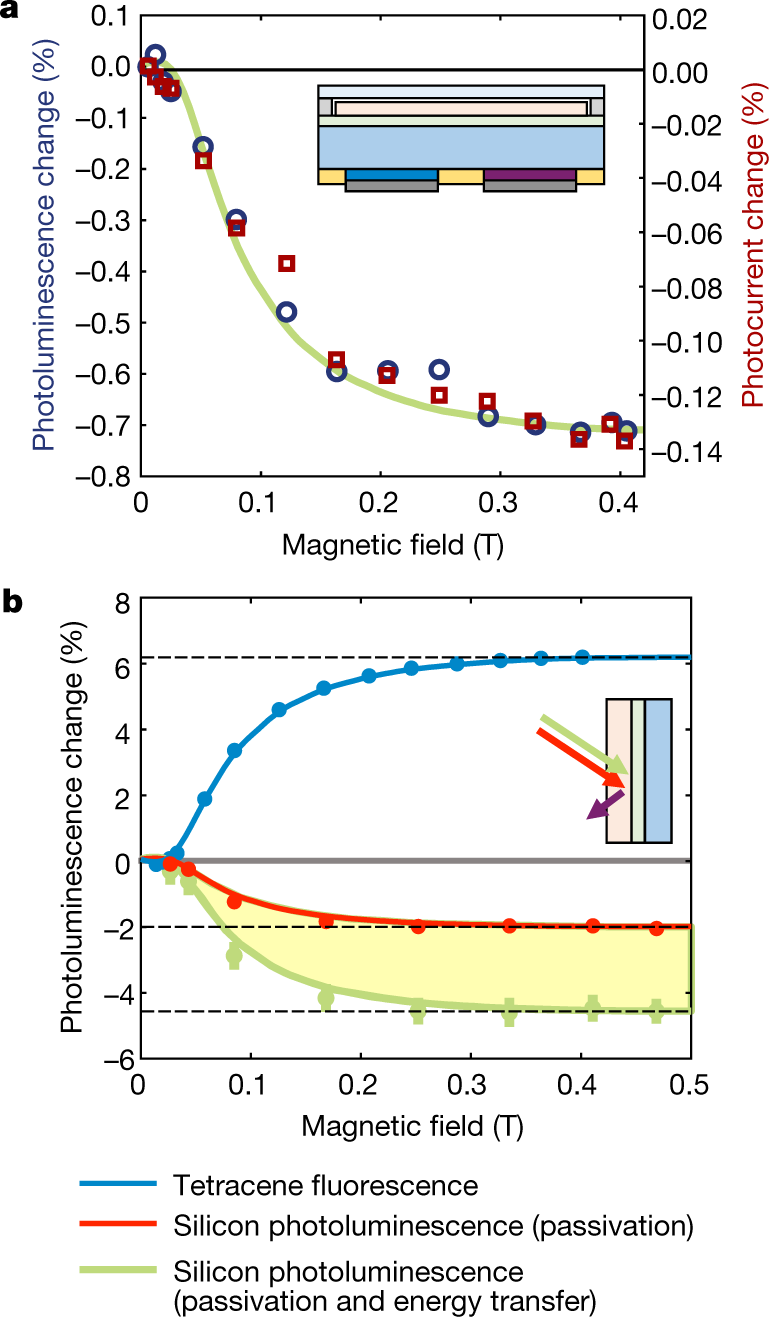
The caption:
The down conversion of high energy photons to photons in this useful bandwidth is most interesting. High energy radiation is generally available: We can make all we want although in general we are too stupid to want to make it even though there is an excellent argument that we need it.
As for this latest "renewable energy" "breakthrough," it offers a useful exercise in understanding how "renewable" so called "renewable energy" actually is.
Hafnium is found as an impurity in all zirconium ores, generally at a concentration (with respect to zirconium) of around 3%, and must be removed from them in order to utilize zirconium in nuclear reactors. Hafnium free zirconium is also obtainable as a fission product, but very little hafnium (if any) is synthesized in nuclear reactors, although one can imagine tiny amounts being made in long lived "breed and burn" reactors that can run for decades without refueling.
Hafnium is considered an "endangered" element, one that may be totally depleted for future generations:

One could argue that the amount of hafnium in these solar cells is tiny, but that claim will not stand up to an effort to scale this low energy to mass type system to a scale of tens of exajoules. Of the less than 11 exajoules from so called "renewable energy" as a whole, solar energy is trivial in the trivial system with respect to wind energy, which represents the bulk of the 11 exajoules.
Nor is this chemistry remotely clean. Chloromethane is made from dangerous natural gas, and hydrofluoric acid is not good for you. Although I am personally fond of hydroflouric acid's use in nuclear processes, these processes require relatively trivial amounts, since the energy to mass ratio of nuclear energy is huge, whether compared to gasoline, to coal and to dangerous natural gas or to far less concentrated forms of energy, the mass intensive, and thus environmentally suspect solar industry.
Have a nice "4th of July" evening. Don't get burned by fireworks.
nocoincidences
(2,230 posts)Solar panels will be installed on my roof next week and hooked into Dominion Electric so that any overage I generate will be carried over to the next month. Big savings each month
NNadir
(33,565 posts)Twenty five years from now - quite possibly less - they will be electronic waste. I'm sure that someone in the third world, which of course may move here will be very pleased to handle them.
The people who are involved in the clean up of the waste that was generated from making them of course are here today and we have a term to describe them: Poor people.
I'm not a fan of solar cells, since your big savings have a cost even if you don't pay it. I personally find this cost environmentally and morally unacceptable.
Overall the solar industry has proved completely and totally useless in addressing climate change. The more than one trillion dollars squandered on the last ten years on this stuff has done nothing, absolutely nothing, to address climate change. The rate of carbon dioxide increases (the second derivative) is now the highest ever recorded, 2.4 ppm, approaching 2.5 ppm, per year.
The data is in the OP. Here's a graphic from the Mauna Loa website showing the general trend since the "renewable energy will save us" fantasy took hold at the turn of the century:
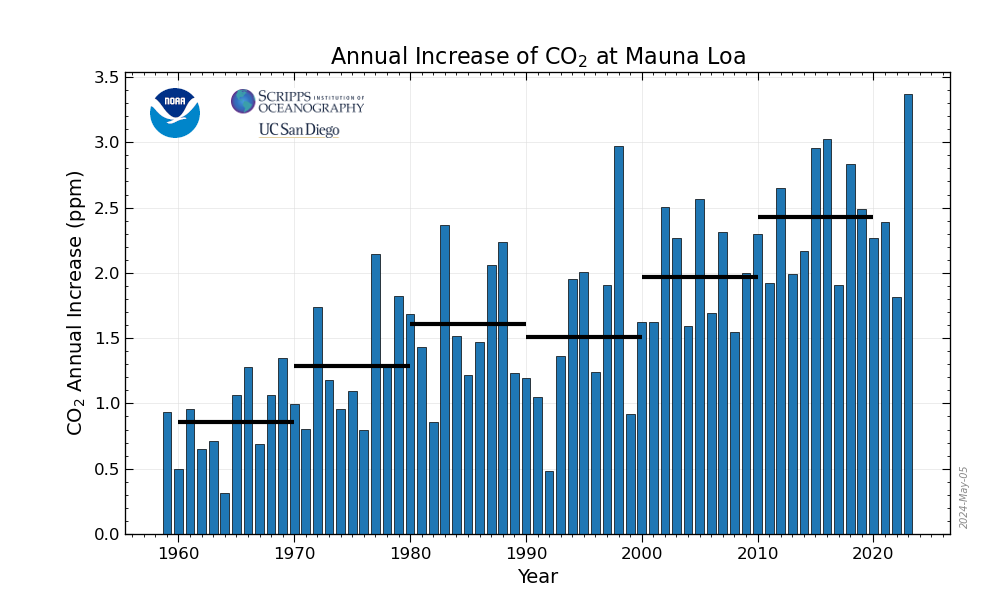
Are things getting better or worse?
Have a pleasant holiday weekend.
Eko
(7,369 posts)nocoincidences
(2,230 posts)Did you notice Fukushima?
NNadir
(33,565 posts)Last edited Fri Jul 5, 2019, 07:23 AM - Edit history (1)
...in the last ten years from air pollution.
Why is that?
It's in a scientific publication that no one needs to go to a scientific library to read:
I have zero respect for people who imagine that one death from radiation at Fukushima is equal to the seven million people who will die this year from air pollution.
However, I am spectacularly unsurprised to hear about this rhetoric, even though in 100% of the cases where people whine about Fukushima, I know more about that accident then they do.
Suppose for one second that as many people died from radiation as died from seawater in the Sendai/Fukushima earthquake, 20,000.
At the death rate from air pollution at a rate of seven million people per year, which reduces to 19,000 people a day, 800 people per hour, 13 people per minute, roughly, this year we would have 365 Fukushimas this year because we didn't have nuclear power replacing all polluting dangerous fossil fuels.
How come the same people who in their tiny imaginations think that radiation deaths are so important that we should ban nuclear power because of Fukushima are spectacularly uninterested in banning coastal cities because of Tsunamis?
We of course have lost tens of thousands of people this week in Europe from heat, which exceeded the 2003 event by far, in which 70,000 people died.
Death toll exceeded 70,000 in Europe during the summer of 2003.
(Jean-Marie Robain et al, Comptes Rendus Biologies Volume 331, Issue 2, February 2008, Pages 171-178).
I have heard all about Fukushima, endlessly to the point of nausea, and know all of the details, about which I've researched a read extensively.
What do you know about climate change?
One of the world's great climate scientists, Jim Hansen, authored a widely read and cited scientific paper on the topic of the effect of nuclear energy on human lives, and concluded, irrefutably, that nuclear energy saves lives:
Prevented Mortality and Greenhouse Gas Emissions from Historical and Projected Nuclear Power (Pushker A. Kharecha* and James E. Hansen Environ. Sci. Technol., 2013, 47 (9), pp 4889–4895)
Any fool can read it, but most fools won't.
However it follows from it that in effect, anti-nuke selective attention kills people.
Nuclear energy need not be without risk; it need not be perfect to be vastly superior to everything else. It only needs to be vastly superior to everything else, which it is.
I'm a scientist, not an airhead.
Thanks for your comment; I'm afraid I won't have the pleasure of reading any more of them, since I now make it a practice to expand my ignore list here to include anyone who asks me if I know about Fukushima. Of course I know all about it, but I know about many other things as well. Their selective attention, and inattention to reality upsets me, and there's no point in engaging these sort of people at their level.
cstanleytech
(26,332 posts)NNadir
(33,565 posts)to supply all of the world's energy for several centuries.
We may add a few centuries if we recover the mined but dumped thorium from the lanthanide industry, which provides lanthanides for wind turbines, electric cars, and many types of electronic devices.
I covered, in some detail, with 59 references, a small subset of available references, the topic of whether uranium is exhaustible by any human technology here:
Sustaining the Wind Part 3 – Is Uranium Exhaustible?
It is not exhaustible. Since I wrote that piece in 2015, many thousands of papers, along the same lines for uranium extraction and removal from dilute aqueous matrices have been published, over 7,000 for seawater alone according to Google Scholar.
The ocean contains just under 5 billion tons of uranium, constantly cycled through the crust and mantle, and I covered this cycle in some detail in that article.
To supply 600 exajoules annually - a little more than what humanity consumes now each year - requires about 7,200 tons of uranium, converted in process in modern "breed and burn" type reactors to plutonium. These "breed and burn" reactor types, Nuscale, Terrapower, etc, etc, etc. are designed to not be refueled for decades and can be started by a relatively small critical mass of plutonium. The depleted uranium in the United States alone is about half a million tons.
Wherever seawater is processed, whether for desalination or simply for cooling, uranium can be recovered from it, economically at about 3 or 4 times the cost of terrestrial ores. The intense energy density of uranium means that the cost of the fuel, particularly if one eliminates enrichment as one can do, means that the price of uranium is meaningless. Current prices are the equivalent of gasoline at a fraction of a cent per gallon in terms of energy content.
I note that by use of high temperatures, one can raise the thermodynamic efficiency of nuclear reactors to a very high level, and, in cases where supercritical seawater is so obtained, effectively clean the ocean of microplastics as well as recover atmospheric carbon dioxide extracted from the atmosphere in various forms.
cstanleytech
(26,332 posts)posts you have been pretty harsh towards electric cars due to the materials needed to produce the batteries but if we are to rely on nuclear it stands to reason we are going to need those especially once oil gets to low in volume to be a viable fuel.
NNadir
(33,565 posts)I reject the notion, so entrenched in modern thinking that the car CULTure can be made sustainable.
The car is the primary example of why distributed energy is a bad idea, despite all of the endless claims to the contrary.
I don't just reject electric cars; I reject cars.
I note that there are many people on this planet, billions, right now, who have led and are leading useful and productive lives without cars. Billions of people lived useful and productive lives before 1900.
Therefore the idea that we need cars is somewhat absurd; and yet I'm often called on this, with people asking "how can nuclear energy keep our cars?"
Now, if it happens that we insist on a stratified society that will ultimately choke to death on the type of inequality we see today, it is certainly possible, and has been demonstrated on an industrial scale, that if one has carbon and water, one can make every commodity chemical currently found in oil. This process is called the Fischer-Tropsch process and was first demonstrated in Nazi Germany during the Second World War, and later in South Africa. Eastman had a plant in Tennessee, or perhaps has a plant there that does something very close to make, if I recall correctly, methyl acrylate.
These were and are systems that use coal as the carbon source, but as I have posted many times in various places around the internet, it is possible to reduce carbon dioxide, including thermochemical splitting, to carbon monoxide with heat and/or (worse thermodynamically) electricity. Even casually leafing through scientific journals, one can stumble across papers along these lines fairly regularly.
If one has carbon monoxide, one can make pure carbon, a quasi synthetic coal, via a reaction known as the Boudouard reaction, which is essentially a disproportionation of carbon monoxide (where it reduces and oxidizes itself simultaneously) into carbon and carbon dioxide, the latter subject to further reduction.
This said, one can imagine a sustainable world where a certain subset of self propelled vehicles, tractors, ambulances, perhaps delivery trucks might be necessary on some level.
To the extent we either want or need self propelled vehicles, we should not make the mistake of either making batteries or synthetic gasoline or diesel fuel since these are both unacceptably dirty and responsible for millions of deaths each year. A clean and sustainable fuel is dimethyl ether.
It is described here: The International Dimethyl Ether Association
It's rather technical, but this fuel is accessible from nuclear heat.
To obtain carbon, we must utilize two sources, one being biomass, particularly with respect to oceanic biomass, in forms that can recover phosphorous from the oceans since we are hell bent in these times on rendering sea birds extinct with moronic ideas about wind turbines at sea, which will besides destroying seabirds will also add to the plastic load, metal and oil contamination of the oceans and will do nothing to address climate change.
I note that the huge dead zone at the Mouth of the Mississippi caused by the excess use of so called "renewable energy" to power cars, corn ethanol grown in Iowa, is a rich field of carbon; it is the decomposition of carbon from algae that causes the oxygen depletion that kills the Mississippi Delta's ecosystem. I have been thinking about this carbon source for many years, but never saw it addressed until recently I stumbled across a paper by Chinese scientists proposing exactly the same thing for a Chinese lake being destroyed by eutrophication.
But overall, the best source of carbon dioxide, one we must use if we have any hope of reversing climate change, is the carbon dioxide absorbed into the ocean. An interesting scientist working in the US Naval Research Lab, Heather Willauer, has demonstrated this to make Naval Jet fuel, albeit by a process that is electrochemically driven, using electricity. using seawater as the carbon source.
She's built pilot plants to do this. She claims she can make jet fuel at around $6/gallon with current electrical technology.
I'm quite sure that a number of other processes, better processes, can be developed. The issue is not substance, it is energy. Thermochemical processes are all superior to electricity in thermodynamic terms, since the production of electricity assumes huge thermodynamic losses, around 30% in the best case, but more like 65% in current industrial practice. Certain kinds of flash distillations (at reduced pressure) of seawater to obtain fresh water produce carbon dioxide as a side product greatly enriched in that gas with respect to its concentration in air, and to the extent this carbon dioxide is captured and reduced, it is sequestered, particularly if rendered into solid forms like carbon fibers, metal carbides (refractories essential for use in devices like reformers), graphene, long use polymers, etc, etc.
We don't simply need to stop dumping carbon dioxide. Our inattention to the issue coupled with wishful thinking and selective attention have made it a far more challenging task, which is to remove carbon dioxide from the atmosphere. I think it may be possible, but not quite so long as we spend our time daydreaming and/or whining about how we can't change anything, including but not limited to the car culture, but must live in a consumer sybaritic hell. Very few of us can live at all, perhaps none of us, should we not break out of that assumption.
When I say "we," I am speaking of humanity in general. I am not personally involved anymore. I will personally die soon enough, disgusted with what my generation has left for future generations, a large portion of that disgust focused on the idea that we "needed" our cars.