NNadir
NNadir's JournalIt's like Gabriele Sadowski always says,...
"...The appropriate equations can easily be written for any potential function as
 "
"
Easily...
She's such a kidder, that Gabriele...
Perturbed-Chain SAFT:? An Equation of State Based on a Perturbation Theory for Chain Molecules (Joachim Gross and Gabriele Sadowski, Industrial & Engineering Chemistry Research 2001 40 (4), 1244-1260)
No threat.
For Father's Day, my son sent me an electronic copy of a book called "Nuclear Corrosion Science and Engineering."
My virus protection software scans every file I download.
So I downloaded a chapter an this popped up: "No threat in Corrosion issues in supercritical water reactor (SCWR) systems."
I don't know about you, but I'm pleased to find that out. I guess I don't have to read the chapter.
Chemist Lisa Jones withdraws from UNC faculty search for Pulitzer-winning journalist's tenure denial
Tenure was denied to Nikole Hannah-Jones, who is the Pulitizer Prize Winning author of the 1619 Project, which discusses the role of historical slavery in modern African American lives. Lisa Jones and Nikole Hannah-Jones are not related.
This news item is from here: Chemist Lisa Jones withdraws from UNC faculty search over Pulitzer-winning journalist's tenure denial.
Subtitle:
An excerpt:
The chemistry department had spent two years trying to recruit Jones, well known for her work in structural proteomics and currently an associate professor in the Department of Pharmaceutical Sciences at the University of Maryland School of Pharmacy. However, in her letter to the department, Jones said she could not see herself accepting a position at a university where the Board of Trustees’ decision to intervene and refusal to hire Hannah-Jones with tenure stands. Hannah-Jones, a MacArthur Foundation “genius” grant winner best known for the 1619 Project, a New York Times Magazine initiative that underlines the role of slavery and Black Americans in US history, is set to join the UNC faculty in July as the Knight Chair in Race and Investigative Journalism at the Hussman School of Journalism and Media.
Jones, who is Black, declined to be interviewed for this article but emailed the following statement: “Hearing of the delay of Nikole Hannah-Jones’ tenure decision led me to reconsider whether the environment at the University of North Carolina (UNC) would be conducive to the achievement of my academic aspirations, which include promoting diversity, equity, and inclusion. While I have never met Ms. Hannah-Jones, as a faculty member of color, I stand in solidarity with her and could not in good conscience accept a position at UNC. This situation is indicative of a broader issue within academia where faculty of color face several obstacles and are less likely to gain tenure.”
Chemistry department chair Wei You sent a letter to Chancellor Kevin Guskiewicz and other administrators that said Hannah-Jones’s tenure case is “already having a chilling effect on future hiring at UNC, particularly from under-represented groups.” You urged the chancellor and trustees to fix the situation.
During its 203-year history, UNC’s chemistry department has only recruited three Black faculty to tenure-track positions; Jones would have been a fourth.
Hannah-Jones weighed in by tweet: “I’ve never met this sister, Dr. Lisa Jones, but the solidarity shown me by Black women in particular during this crucible is something I will never forget...”
Scientists do need to speak out on issues in ethics, and Dr. Lisa Jones is responding in that spirit.
Obama and a Form of Liver Cancer.
A while back in this space, I noted that the science policies of the Clinton administration probably were the drivers for the rapid response to Covid-19. Without the scientific infrastructure for gene mapping for which he pushed, the Covid vaccines would still be years away:
It just occurred to me who the President who did the most to fight Covid was.
I'd now like to point out how the Obama administration built on that legacy, albeit nothing quite as immediately dramatic.
This news item appeared in my work email a few days ago, in one of the scientific newsfeeds to which I subscribe: Team Finds Compounds Capable of Killing Lethal Liver Cancer Cells
Some excerpts:
Sanford M. Simon and his group understood that patients dying of fibrolamellar could not afford to wait. "There are people who need therapy now," he says. So his group threw the kitchen sink at the problem and tested over 5,000 compounds, either already approved for other clinical uses or in clinical trials, to see whether any of the compounds could be repurposed to treat fibrolamellar. The team ultimately discovered a few classes of therapeutics that destroy fibrolamellar tumor cells growing in mice. Their findings are published in Cancer Discovery.
"We decided to be completely agnostic about what we thought would work--we tried everything," says Simon, head of the Laboratory of Cellular Biophysics. "To our surprise, we found a few compounds that work really well..."
...The findings suggest that it may be unnecessary to screen new cancer drug candidates in cells grown in mice before testing them on human cells--an extra step that can cost cancer researchers many months. Given these results, physicians may soon be able to biopsy cells from a patient's tumor, subject those cells to a bevy of drug candidates until they find the most effective compound for that specific patient, and have a treatment plan ready in a matter of days--potentially transforming the landscape of precision medicine...
Advances in precision medicine
...Simon's recent work was inspired by the 2015 precision medicine initiative begun in the Obama administration, which promised to change the face of medicine with a targeted approach, tailored to a patient's unique genetic composition, lifestyle, and environment.
"You don't want to give everyone with a limp the same treatment--you want it 'precisely targeted' based on whether they have twisted their ankle, broken a bone, or just have a splinter," says Simon...
I added the bold referring to the Obama Administration.
Wise investments in science do not pay off immediately, but they do provide for a better future.
Three Generals Reconsider Ulysses S. Grant and Robert E. Lee.
I watched this conversation, broadcast by CSPAN history this afternoon, between three American Generals, reevaluating the legacy of Ulysses S. Grant and Robert E. Lee, the context of the discussion focusing especially on racism in the US Army, West Point,
Retired Generals David Petraeus, Dana Pittard and Ty Seidule discussed military bases named for Confederates, various memorials to Robert E. Lee on the campus of West Point and whether Grant should posthumously be made a five-star general. The Grant Monument Association provided this video. One can view it at the link below and I recommend it highly for anyone interested in the history of racism in this country.
General David Petraeus is famous for the Iraq war of course, and is a graduate of West Point, as is Brig. General Dana Pittard, who is African American, who discusses his experience as an African American officer in the US Army beginning with his years at West Point.. General Ty Seidule is the former head of the military history department at West Point.
There was much discussion of General Siedule's book, ROBERT E. LEE AND ME A Southerner's Reckoning with the Myth of the Lost Cause. This is definitely on my reading list; had I known about it I would have requested it from my sons as a Father's Day gift.
General Siedule got his undergraduate education at Washington and Lee University in Virginia and he describes his trajectory from being a student at a University where there is a chapel dedicated to Robert E. Lee, festooned with Confederate flags - flags that General Pittard describes as being to him the equivalent of Nazi flags - to a serious historian who has recognized that Robert E. Lee was a traitor to his oath and his country. General Siedule discusses how US Army bases, such as Fort Hood, Fort Benning and Fort Bragg came to be named after traitors to their country who actually killed US soldiers. He now is on the commission to remove the names of all of the Confederate traitors from the names of US Army bases.
I recommend watching this conversation highly. It is available on line here:
Generals Grant and Lee Reconsidered.
CSPANs text describing the video:
Generals Grant and Lee Reconsidered
To mark the 199th anniversary of Ulysses S. Grant’s birthday, the Grant Monument Association hosted a discussion about Grant and Lee’s legacy. Retired Generals David Petraeus, Dana Pittard and Ty Seidule discussed military bases named for Confederates, various memorials to Robert E. Lee on the campus of West Point and whether Grant should posthumously be made a five-star general. The Grant Monument Association provided this video.
CA Extreme Temperatures, Electricity Demand Peaks, Timing of So Called "Renewable Energy" peaks.
Extreme heat being experienced in California and it is worth considering how so called "renewable energy" is faring on addressing the electricity demand associated with the need for cooling. I have been following this extreme weather event as well as sources of electricity over the last few days utilizing the real time data at the CAISO website, which details in graphics and text how power is being generated on the California electrical grid.
The data is available at the CAISO website: CAISO Website.
CAISO uses the 24 hour clock, (military time).
Parts of this post will include verbatim language from the previous two posts in this series, particularly with respect to an interesting note from the scientific literature on human survivability in extreme heat situations listing some references - not comprehensive by any means - to the death tolls associated with similar events.
The two earlier posts are here:
CAISO: Heat Wave in CA,So Called "Renewable Electricity" Matched Gas Electricity for 10 Minutes.
Looking at CAISO demand and supply of electricity during extreme California temperatures.
For the record, I am an ex-Californian (but not a native of that state). I lived there, on an off, for a total of about 13 years, leaving for good in 1993. For many of those years, I heard about how wonderful so called "renewable energy" was, and observed many bills that were passed by the legislature and signed by the Governors of the State setting goals for a "renewable nirvanas" the state was going to become with the "...by 'such and such year.'" This historical "...by 'such and such year'" language is still in use today, even as the planetary atmosphere is collapsing, as the current events in California show, not "...by 'such and such year,'" but now.
I pulled up the daily hourly temperature data from some random inland California cities that I remember visiting or passing through:
Indio's high on 06/18/21 was 119°F, (48°C), recorded at 17:53 (2:53) pm. From 11:45 PDT to 19:00 PDT (17:00) the temperatures were above 110°F, (43°C), and from 8:45 to 22:10 PDT (10:10 PM) the temperature was above 100°F, (38°C).
Indio is in the Imperial Valley, a major agriculture area, the "salad bowl" of America; the agriculture is supported by irrigation using the rapidly dying Colorado River where all time lows (since the 1930's when it was built) in the level of "Lake" Mead behind the Hoover Dam are being recorded, not by "...by 'such and such year,'" but now. This is not going to be a good year for cheap salads.
Bakersfield's high was 109°F, (43°C) at 17:54 PTD (5:54 pm). This is the same temperature used by the researchers testing human heat endurance in the reference and text I will repeat below from the earlier post. Temperatures in that city were at or above 100 °F, (37°C) from 11:00 PDT to to 18:00 PDT (6:00 PM).
Lancaster's high on 06/18/21 was 109°F, (43°C), recorded at 15:40 PDT (3:40 PM). Temperatures in that city were at or above 100 °F, (37°C) from 9:45 PDT to to 17:56 PDT (5:56 PM).
Pretty much every day my wife and I celebrate the fact that we now live in what, for me, is the best state in the Union, New Jersey. We enjoyed the time we spent together in California in our early marriage, but we now consider that California is rapidly becoming uninhabitable. I lived through California droughts, and always tried to do my part, flushing the toilet with collected shower water from short showers, wearing clothes a few extra days to avoid needing to laundering them, fixing leaks as soon as I saw them, among other things.
No crisis in California I experienced however, is anything like the crisis now being experienced, a climate crisis.
The real time CAISO graphics by the way reset each night at midnight.
It happened that I was up in the middle of the night around 2 am EDT, so I was able to capture the electricity state near the end of the California for nearly the entire day.
California is often presented as a so called "renewable energy" paradise.
We are nearing the summer solstice, and of course, California is a putative solar energy nirvana in particular.
Since I check this website frequently, and have been doing so as we approach the solstice, we can expect that so called "renewable energy" will be dominated by solar production in the early afternoon.
People like to cheer for what so called "renewable energy" does at peaks or to compare brief periods when it is producing more energy than energy production for energy production systems they hate, or pretend to hate. For about 2 hours yesterday in the morning hours, well before the peak heat hours, so called "renewable energy" was producing more than dangerous natural gas. Advocates of so called "renewable energy" love to tell me that they hate dangerous natural gas, which they call "transitional." I know better. They're full of shit. Perhaps they're lying to themselves when they say this, but I'm unimpressed when they try this lie out on me. So called "renewable energy" depends on access to dangerous natural gas; this was true yesterday in California, after decades of jawboning in that State about how so called "renewable energy" would save to day "...by 'such and such year,'" the majority of air conditioners in an extreme heatwave would have had to shut down if California did not have access to dangerous natural gas, lots of dangerous natural gas.
Dangerous natural gas is not clean; it is not safe, and it releases significant amounts of the important dangerous fossil fuel waste carbon dioxide, and leaks for the transport and use of dangerous natural gas releases the second most important climate forcing gas, methane.
Even though the climate change is killing the planet, and in this event and in other areas, California, California was compelled to make things worse because their "renewable energy" fantasy continues, and I contend will always continue, to depend on access to dangerous natural gas.
There is no doubt in my mind that people are being killed by heat this week in California, even if there have been no major power outages and most of the air conditioners in the State or running. Most of the dead will be poor people, not that bourgeois types who have bet the future of humanity on so called "renewable energy," a form of energy with an oxymoronic title inasmuch as the dependence of vast mining of irreplaceable elemental resources means it is inherently not sustainable, give a rat's ass about poor people. They'd rather discuss their Tesla electric cars.
Humans cannot survive temperatures much higher than 120°F (49°C) without drinking copious amounts of water. A small human clinical trial with 10 young healthy male volunteers, evaluated strategies for survival at lower temperatures (43.0 ± 0.5 °C) than we're seeing in Indio, in a 90 min trial, in the absence of air conditioning:
Intermittent wetting clothing as a cooling strategy for body heat strain alleviation of vulnerable populations during a severe heatwave incident (Song, Wang, Zhang, Journal of Thermal Biology 79 (2019) 33–41).
The subjects, all men in their early 20s dressed in light clothing, all lost about 120 - 130 grams of water to evaporation and produced about 350 grams of sweat in this period.
The introduction to that paper had some fun text about the death toll associated with heat waves, albeit assuredly not in any way a comprehensive accounting:
In extremely hot environments (Tair? 40 °C), people like the poor and the homeless in backward areas do not have a chance to access air-conditioning. Hence, they have a high risk of suffering heat stress during prolonged heatwave incidents. In fact, statistical data showed that those populations account for a large proportion of heat-induced death tolls (Åström et al., 2011, Gronlund, 2014, Gubernot et al., 2014). Besides, extreme heatwaves put strains on the electrical power grid and cause power outages in some regions which renders electrically powered cooling devices (e.g., air-conditioning, electric fans and water pumps) useless...
Power outages in these conditions can kill a person.
Of course, people don't often discuss the death toll associated with heat waves. Most people, in my experience, would rather talk about the 2011 Earthquake and Tsunami in Japan in which 20,000 people died from seawater, although the deaths from seawater are in no way as interesting as the possibility that someone some day somewhere may die from radiation that leaked from nuclear reactors destroyed by the Tsunami.
I'm frequently told that nuclear power is "too dangerous," by people who apparently believe that climate change is not "too dangerous." By contrast, I've been hearing for most of my adult life - I'm decidedly not young - that so called "renewable energy" will save the day. It hasn't saved the day and it isn't saving the day, but in these times, we like to substitute faith for facts.
Blind faith, and even cognizant faith, often involves lying, lies we tell ourselves, lies we tell other people.
And of course, we live in an age of the celebration of the lie, both on the right wing, and regrettably, on our side of the aisle.
There is one nuclear plant left in California, Diablo Canyon (2 reactors). For most of the day yesterday it was producing about 2,265 MW of electricity in two small buildings, more than all the wind turbines in California were producing between the hours of 4:50 PDT and 17:40 PDT (5:40 PM). The reactor came on line 36 years ago, and is functioning fine. It's reliable and predictable. No one has been killed by pollution produced by the Diablo Canyon Nuclear Plant. I contend that the used nuclear fuel stored there, all of it on site, will be a valuable resource for future generations that will be less stupid than mine has been.
Some graphics from the CAISO site relating to the period that all the wind turbines in California were producing less energy than all the wind turbines in California, including many hours of extreme temperatures:


According to the California Energy Commission the "capacity" of wind energy is 5,983 MW, more than 40 years after the State began authorizing the destruction of huge stretches of pristine wilderness for wind farms, beginning in the late 1970's at Altamont. I lived in California at the time, and I confess, with more than a little contempt for my youth, that I cheered for the wind turbines in California. I will not forgive myself, nor should I.
People who embrace the lie that so called "renewable energy" is sustainable and environmentally benign like to point to peak capacity of wind (and solar) installations as if they are the same as peak capacity at nuclear plants. At 2265 Megawatts, the Diablo Canyon nuclear plant, about to be destroyed out of a sense of contempt for the collapse of the atmosphere by people who can't think straight, was functioning very close to 100% of its rated capacity. By contrast, all of the wind turbines spread over vast areas now rendered into industrial parks in the State were operating for the period they were producing less energy than the nuclear plants were operating at less than 37% capacity.
I'd like to point to the fact that at 4:50 PDT solar energy was sucking energy out of the grid rather than adding to it. This was related to the fact that there is a period of time known as "night" every 24 hours, predictably and reliably. The fact that solar energy was sucking power out of the grid is involved with the requirement that copper wires are required to connect all of this so called "renewable energy" garbage to the grid, all of which will become landfill within the next 25 years.
An interesting fact about California energy supplies is that in the case of electricity it has been found out that all these wires connecting all this unreliable crap sometimes overheat. Huge fires have resulted, because the dryness caused by climate change, climate change being connected with the faith based fashion for so called "renewable energy" has had no effect on slowing climate change at all - the rate of degradation of the atmosphere during these years of world wide enthusiasm for so called "renewable energy" is accelerating, not decelerating. As a result, the California power grid has been shut down at times of high heat and high winds by its operators to prevent fires.
If you think all these wires are not connected with enthusiasm for so called "renewable energy," you're not paying attention. A recent scientific paper in a scientific journal I regularly read reported that way to address what the Germans call "Dunkelflaute," wind and solar droughts that mimic water droughts, has proposed that the way to address Dunkelflaute in California is even more mining of more copper to make more wires:
Wind and Solar Resource Droughts in California Highlight the Benefits of Long-Term Storage and Integration with the Western Interconnect (Katherine Z. Rinaldi, Jacqueline A. Dowling, Tyler H. Ruggles, Ken Caldeira, and Nathan S. Lewis Environmental Science & Technology 2021 55 (9), 6214-6226)
Again, unfortunately the last nuclear plant in California is about to close because of appeals to ignorance. That will raise the dependency of gas on California. No replacement of this valuable resource, which is at this exact point, producing more energy than all the wind turbines in the entire state, this without turning vast tracts of wilderness into industrial parks, is planned.
The nuclear plant will be replaced by dangerous natural gas. There will be lots of outright lies told to the contrary, but the plant will be replaced by dangerous natural gas.
With the climate induced failure of the Lake Mead/Hoover Dam hydroelectric system expected this summer, large hydro will also be replaced by burning dangerous natural gas. So much for making more and more and more of California's electricity supply dependent on good weather, even if the State is known for "good weather," which ironically enough, includes an absence of rain.
Some more graphics downloaded from the CAISO website in the early morning hours of EDT about the late evening hours PDT:
The solar energy peak power production:

Peak power demand in California, which was 40,751 MW, recorded at 17:53 PDT, (5:53 PM). This graphic shows the grid at 17:50 (5:50 PM) :

This graphic, part of which, the exact number so called "renewable energy" portion, was mistakenly cut off in the sloppy wee hours of the morning when I was suffering from insomnia, shows pretty much all of the energy sources at the peak hour. By eyeballing the graphics, one can see that so called "renewable energy" was producing about 12,000 MW, and falling, as the sun went down. Except for the lone nuclear plant and the large hydro which is now due to become as unreliable as solar and wind in Dunkelflaute events, almost all of the electricity in California was produced by burning dangerous fossil fuels and dumping dangerous fossil fuel waste, including but not limited to carbon dioxide, directly into the planetary atmosphere where it is destroying that atmosphere:

The supply position at around the hour I gave up in disgust and tried to go back to bed.

There is a serious risk of California, particularly Southern California, becoming uninhabitable, particularly with respect to the effect of climate change, to which dangerous natural gas is a contributor, on water supplies. You may think I'm being extreme here, but I don't think so.
We're kidding ourselves if we think we're doing anything to address climate change.
History will not forgive us; nor should it.
I wish all fathers a happy "Father's Day" tomorrow. Both my sons will be with me tomorrow to celebrate our relationship.
CAISO: Heat Wave in CA, So Called "Renewable Electricity" Matched Gas Electricity for 10 Minutes.
This is a follow up of my thread of yesterday, about the extreme heat being experienced in California and how so called "renewable energy" is faring on addressing the electricity demand associated with the need for cooling.
That thread is here: Looking at CAISO demand and supply of electricity during extreme California temperatures.
Yesterday I focused on the weather in San Bernadino, where the high temperature yesterday was 102°F (38°C) and mentioned Indio, California, one of California's fastest growing cities, albeit with a relatively small population for California of around 80,000. Today in San Bernardino, the predicted high will be again 102°F (38°C) at 3 pm. Indio is cooling down compared to yesterday, where temperatures of over 120°F (49°C). Today's high will "only" be 118°F (48°C).
Humans cannot survive temperatures much higher than 120°F (49°C) without drinking copious amounts of water. A human trial with 10 young healthy male volunteers, evaluated strategies for survival at lower temperatures (43.0 ± 0.5 °C) than we're seeing in Indio, in a 90 min trial, in the absence of air conditioning:
Intermittent wetting clothing as a cooling strategy for body heat strain alleviation of vulnerable populations during a severe heatwave incident (Song, Wang, Zhang, Journal of Thermal Biology 79 (2019) 33–41).
The subjects, all men in their early 20s dressed in light clothing, all lost about 120 - 130 grams of water to evaporation and produced about 350 grams of sweat in this period.
The introduction to that paper had some fun text about the death toll associated with heat waves, albeit assuredly not in any way a comprehensive accounting:
In extremely hot environments (Tair? 40 °C), people like the poor and the homeless in backward areas do not have a chance to access air-conditioning. Hence, they have a high risk of suffering heat stress during prolonged heatwave incidents. In fact, statistical data showed that those populations account for a large proportion of heat-induced death tolls (Åström et al., 2011, Gronlund, 2014, Gubernot et al., 2014). Besides, extreme heatwaves put strains on the electrical power grid and cause power outages in some regions which renders electrically powered cooling devices (e.g., air-conditioning, electric fans and water pumps) useless...
Power outages in these conditions can kill a person.
Of course, people don't often discuss the death toll associated with heat waves. Most people, in my experience, would rather talk about the 2011 Earthquake and Tsunami in Japan in which 20,000 people died from seawater, although the deaths from seawater are in no way as interesting as the possibility that someone some day somewhere may die from radiation that leaked from nuclear reactors destroyed by the Tsunami.
I'm frequently told that nuclear power is "too dangerous," by people who apparently believe that climate change is not "too dangerous." By contrast, I've been hearing for most of my adult life - I'm decidedly not young - that so called "renewable energy" will save the day. It hasn't saved the day and it isn't saving the day, but in these times, we like to substitute faith for facts, and who am I to argue with lies in the age of popular lies, where the lies we tell others and the lies we tell ourselves are celebrated?
California is often presented as a so called "renewable energy" paradise.
We are nearing the summer solstice, and of course, California is a putative solar energy nirvana in particular.
Real time data is available at the CAISO website: CAISO Website.
Since I check this website frequently, and have been doing so as we approach the solstice, we can expect that so called "renewable energy" will be dominated by solar production in the early afternoon. As of this writing, the current peak solar production in the entire State of California is, as of 12:10, PDT, 11,382 MW, the high, so far for the day. In the whole state, wind power is producing a total of 710 MW. The predicted peak power demand at the CAISO site for 6/17/21 is at 18:10 PDT, (6:10 PM) will be 43,048 MW, as the sun is going down, and with it, solar power production. The current demand for energy 12:20 PDT, is 35,599 MW.
People like to cheer for what so called "renewable energy" does at peaks. At no point yesterday, did all the renewable energy facilities in the entire State of California, ever, for even for a few minutes, match the power produced by burning dangerous natural gas, and dumping the dangerous fossil fuel waste carbon dioxide directly into the planetary atmosphere.
Today however, for a period of about 15 minutes, so called "renewable energy" matched the output of the dangerous natural gas plants in the state in a period between 9:45 and 9:55; at 9:55, all the so called "renewable energy" in the State of California was producing 12,756 MW, exactly equal to what dangerous natural gas was producing.
I'll pause for cheers...
After half a century of wild cheering for so called "renewable energy," it is still - I contend always will be - dependent on access to dangerous natural gas. We. Couldn't. Care. Less.
There is one nuclear plant left in California, Diablo Canyon (2 reactors). It is producing about 2,278 MW of electricity in two small buildings, more than all the wind turbines in California. The reactor came on line 36 years ago, and is functioning fine. It's reliable and predictable. No one has been killed by pollution produced by the Diablo Canyon Nuclear Plant. I contend that the used nuclear fuel stored there, all of it on site, will be a valuable resource for future generations that will be less stupid than mine has been.
Unfortunately this nuclear plant is about to close because of appeals to ignorance. That will raise the dependency of gas on California. No replacement of this valuable resource, which is at this exact point, producing more energy than all the wind turbines in the entire state, this without turning vast tracts of wilderness into industrial parks, is planned.
The nuclear plant will be replaced by dangerous natural gas. There will be lots of outright lies told to the contrary, but the plant will be replaced by dangerous natural gas.
Dangerous natural gas is not clean; it is not safe, and it releases significant amounts of the important dangerous fossil fuel waste carbon dioxide, and leaks for the transport and use of dangerous natural gas releases the second most important climate forcing gas, methane.
Graphics from the CAISO website for power production in California as of 12:15 PDT, 06/17/21:
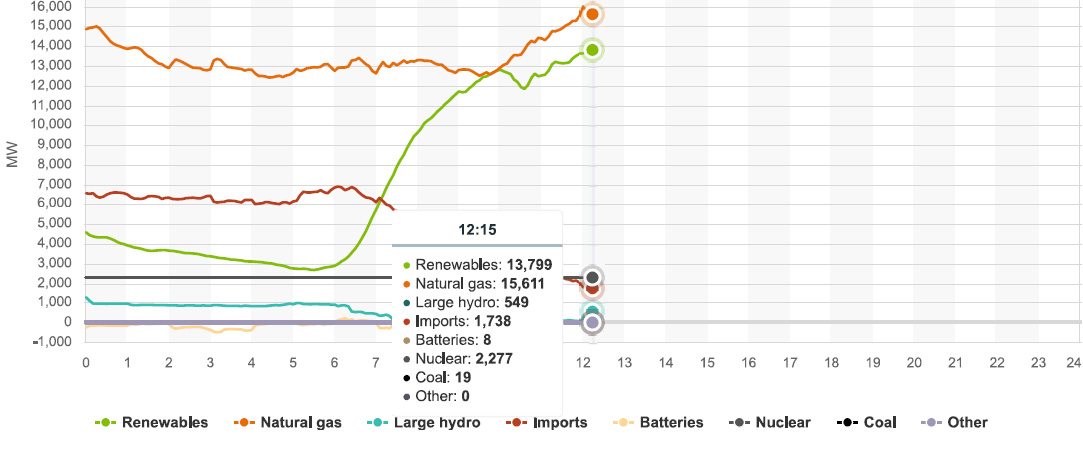

There is a serious risk of California, particularly Southern California, becoming uninhabitable, particularly with respect to the effect of climate change, to which dangerous natural gas is a contributor, on water supplies. You may think I'm being extreme here, but I don't think so.
We're kidding ourselves if we think we're doing anything to address climate change.
History will not forgive us; nor should it.
Looking at CAISO demand and supply of electricity during extreme California temperatures.
Temperatures in parts of California today will exceed 105°F (40°C) and in places, Indio for example, will reach around 120°F (49°C). Air conditioning will be cranking up for sure, and will be working at lower efficiency.
We are nearing the summer solstice, and of course, California is a putative solar energy nirvana.
Real time data is available at the CAISO website: CAISO Website.
A short while ago, I downloaded some graphics.
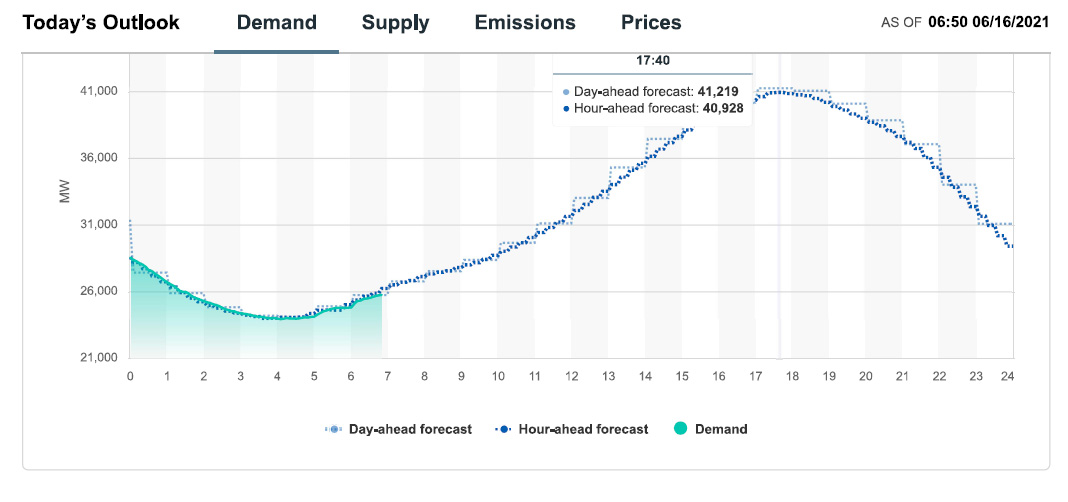
Demand and demand forecast for 06/16/21:
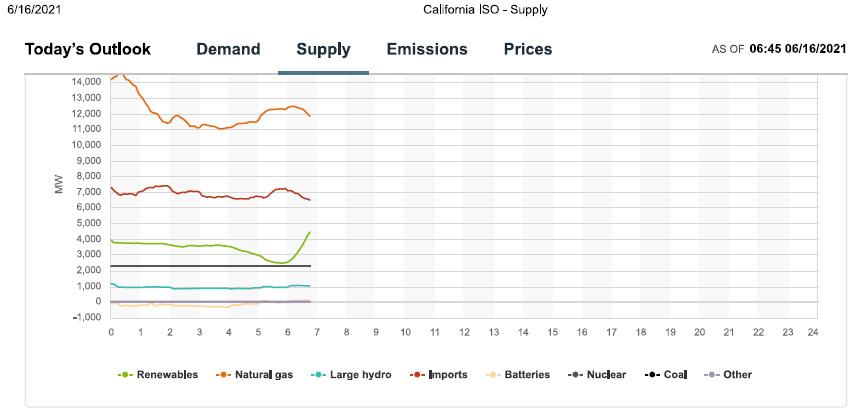
Overall Energy Supply
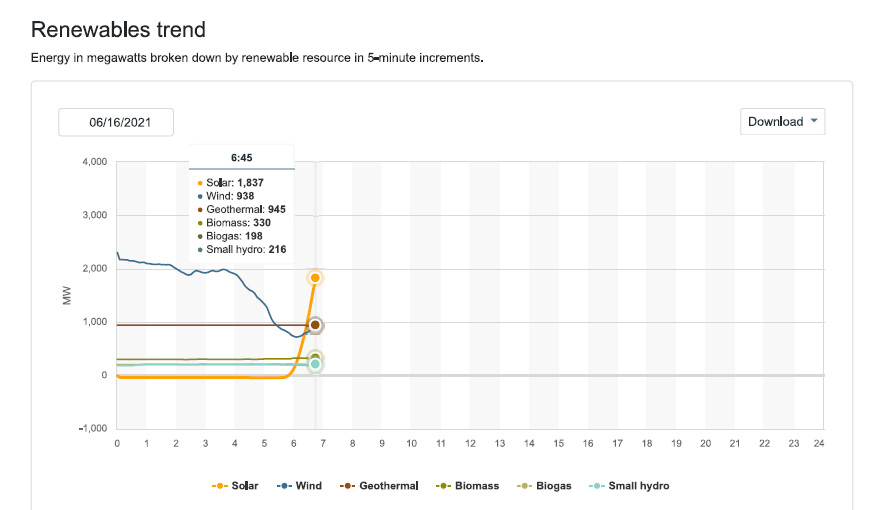
Since I check this website frequently, and have been doing so as we approach the solstice, we can expect that so called "renewable energy" will be dominated by solar production in the early afternoon, which should approach peak power of 13,000 MW.
Impressive, no?
Well, demand will peak as the sun falls, so there's that.
Not much wind is blowing this morning. Maybe it will change, who knows. When and if is not known.
Gas was dominating when I downloaded the graphics, and it will dominate near the peak.
Some people, me for instance, believe the regular occurrence of these kind of high temperature weather events is connected with climate change.
After half a century of wild cheering for so called "renewable energy," it is still - I contend always will be - dependent on dangerous natural gas.
There is one nuclear plant left in California, Diablo Canyon (2 reactors). It is producing about 2,278 MW of electricity in two small buildings, more than all the wind turbines in California. The reactor came on line 36 years ago, and is functioning fine, but well, it's being closed and no replacements are planned in California. (The production of electricity at this plant may fall slightly as the temperature rises in the afternoon, because of changes in thermodynamic efficiency connected with high temperatures, but certainly output will not fall below 2,200 MW). It's reliable and predictable.
Unfortunately this nuclear plant is about to close because of appeals to ignorance. That will raise the dependency of gas on California.
Dangerous natural gas is not clean; it is not safe, and it releases significant amounts of the important dangerous fossil fuel waste carbon dioxide, and leaks for the transport and use of dangerous natural gas releases the second most important climate forcing gas, methane.
There is a serious risk of California, particularly Southern California, becoming uninhabitable. You may think I'm being extreme here, but I don't think so.
We're kidding ourselves if we think we're doing anything to address climate change.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,582
