NNadir
NNadir's JournalRecovering Lanthanides (Rare Earths) From Acid Mine Drainage.
Recovery of Rare Earth Elements from Acid Mine Drainage with Supported Liquid Membranes: Impacts of Feedstock Composition for Extraction Performance Andrew Middleton, Benjamin C. Hedin, and Heileen Hsu-Kim Environmental Science & Technology 2024 58 (6), 2998-3006.Over the last several months, as papers on the subject pile up, happily at an increasing pace, on the subject of removing uranium from aqueous matrices, seawater and groundwater and to a lesser extent, riverine water, where it often occurs, in all three places, naturally as an example of "NORM" (naturally occurring radioactive materials), as well as from anthropogenic sources, uranium mine associated runoff, reprocessing sites generally devoted to weapons manufacture, and coal ash, in which uranium is often a prominent constituent, I have been working on a long post to discuss these many interesting approaches to the idea. Most of these are based on resins, often functionalized with amidoximes, but many other approaches are also discussed in the literature, extending even to proteins found in corals that are known to complex - for what evolutionary purpose I do not know - uranium. I may never get around to publishing it, but I often write posts at DU to force myself to look at the details, as opposed to superficial notation, of particular technologies devoted to a sustainable world, the world which, unfortunately, we do not live, but is, I believe, feasible to create, if unlikely given our mythology driven world culture.
I will not live to see a sustainable world, but hopefully I can hand off some of these ideas, if they have merit, to those who will follow and live with the environmental disaster now well underway.
I often write, in terms of energy, of "process intensification" wherein matrices at high temperatures provided by nuclear fuels are cooled in multiple processes that recover useful products and goals, clean desalinated water, clean portable fluid fuels (DME generally), landfill elimination, and downstream, as a side product, electricity. The idea is that if one can achieve multiple goals with one process or device, one should do so; this is the essence, to my mind, of sustainability.
One of the things we are leaving to the unfortunate generations who will follow us, is mine waste. We of course, consider this waste, but our future generations may have to pick through our garbage merely to survive, as we will have impoverished them. We generally think of mine waste in terms of tailings - some of which contain elements for which we have only recently found important industrial uses, indium for example in zinc mine tailings - but contaminated water is another product of mines.
As far as mines go, things will get much worse before they get better. It is a popular, albeit dangerous, reactionary and rather stupid idea, that so called "renewable energy" is sustainable. It isn't. The term "renewable energy" is essentially an oxymoron: The land intensity and the mass intensity driven by short lifetimes, low energy to mass ratios, and the need for redundancy to address the inherent lack of reliability of the weather - particularly in a period where we have dangerously destabilized the weather - make "renewable energy" not only lack sustainability, but is actively driving the acceleration of environmental degradation. So called "renewable energy" is nothing more than a highly questionable promotion on dependence on mining on land, and now we see, with Norway's recent disastrous decision, at sea.
I am thoroughly convinced of this, despite the unpopularity of my immutable opinion.
It is a huge mistake to confuse popularity with wisdom.
Among the many mined materials on which the so called "renewable energy" industry depends - it is hardly limited to so called "renewable energy" but extends to many other industries - are the lanthanides, in popular parlance, "the rare earth" elements. As is widely discussed in the critical materials issue in academic, industrial and government circles, the world overwhelmingly relies on Chinese mines for these elements, and there is a huge effort, worldwide, to source them elsewhere. The paper at the outset considers recovering them from a well known (and growing) source of water pollution, "acid mine drainage."
From the introductory text of the article:
AMD is generated from the dissolution and oxidation of sulfide minerals in waste rock piles and exposed rock formations produced during mining activities. Water that continuously leaches from these mined areas is enriched in soluble minerals and metals such as REEs, creating a potential source of critical metals. (8,9,14) In the northern Appalachia region of the United States alone, hundreds of abandoned coal mines collectively release 500–3400 t of REE annually. (9,15,16) While the total loading of REE into watersheds is large, the aqueous concentrations of REEs can be relatively low. AMD fluids with the highest concentrations of REE tend to have low pH (i.e., less than pH 4). (9) Even for these low-pH and REE-enriched AMD fluids, the aqueous composition is complex: total REE concentrations are exceeded by up to 5 orders of magnitude by major metals such as Fe, Al, Ca, and Mn. (8) As such, the development of separation processes for AMD and other low-grade feedstocks requires an understanding of the impact of these impurities and other water quality variables on REE recovery processes.
Separations by SLMs are a modification of solvent extraction, where the extraction and stripping processes have been combined into one unit operation. In SLM separations, the feedstock and product solutions are separated by a hydrophobic membrane that has been impregnated with an organic extraction solvent, such as di(2-ethylhexyl)phosphoric acid (DEHPA), dissolved in an oil phase...
Let's stop here. These "SLM" (Supported liquid membranes) are made by soaking a polyvinylidene fluoride, a polymer that is related to teflon, in kerosene containing a lanthanide complexing agent used in the chemical extraction of lanthanides, di(2-ethylhexyl)phosphoric acid (DEHPA). These are placed directly into samples of the contaminated water for the experiments, but not in the flowing mine drainage streams. This information may impact the environmental profile of this particular scheme for using a liquid membrane, but the reader should be aware that there are many other approaches besides "SLM" or even this particular variety of an "SLM;" the important issue is the quantity of elements that can be theoretically (or even practically) recovered from acid mine drainage, not the details.
The authors select, as their test sites shown here:

The caption:
The metals and other species in the water at the seven abandoned mines are described in a table in the paper which follows:
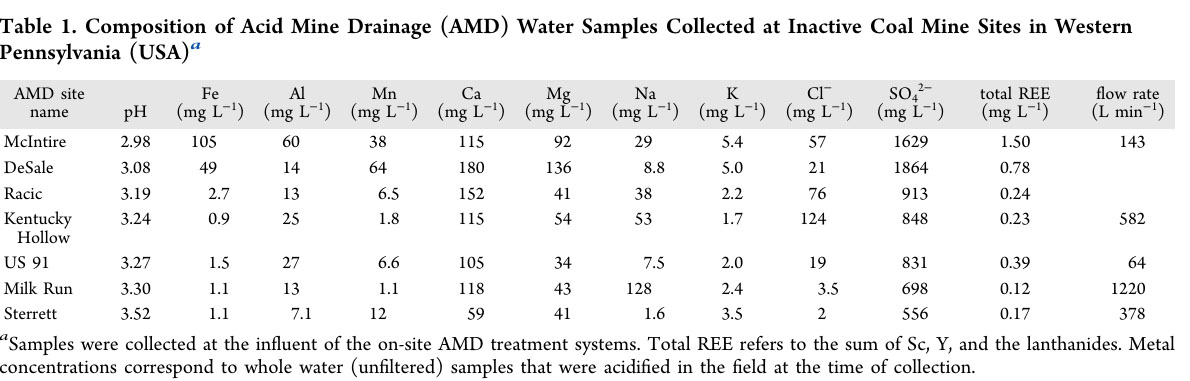
One should note that the amount of lanthanides and the two congeners that are found with them, scandium and yttrium, which are not "f elements" but rather "d elements" but are always found with the lanthanides, is dwarfed by the amount of iron, manganese, and in particular calcium and sodium.
I have loaded the data in this table into a spread sheet, and from the flow rates available for five of the seven mines - flow rates at the Desale and Racic mines are not reported - I have produced an estimation of how much of each of the cationic (metallic) elements flow in a year.
Approximately 3300 tons of iron flow out of the five mines in a year.
Approximately 8300 tons of aluminum flow out of the five mines in a year.
Approximately 2500 tons of manganese flow out of the five mines in a year.
Approximately 49,000 tons of calcium flow out of the five mines in a year.
Approximately 22,000 tons of magnesium flow out of the five mines in a year.
Approximately 39,000 tons of sodium flow out of the five mines in a year.
Approximately 1200 tons of potassium flow out of the five mines in a year.
The fourteen lanthanide elements plus yttrium and scandium total 221 tons out of the five mines in a year.
The supplementary information of the paper gives the breakdown of the 16 elements. Between 45% and 51% are represented by just two elements in the separate mine drainage, yttrium and cerium. Between 63% and 66% are represented by adding neodymium to these two, between 71% and 77% are covered by adding lanthanum to these three, and between 77% and 81% are covered by adding gadolinium.
All of these elements are useful and have markets, but they are not quite as critical as some of the others, in particular, dysprosium.
I have discussed dysprosium here:
Material Flow Analysis of Dysprosium in the United States
...and here:
Uncovering the Key Features of Dysprosium Flows and Stocks in China
If China is concerned about dysprosium availability, everyone should be concerned about it.
Overwhelmingly modern generators and electric motors utilize neodymium-iron-boride magnets, which heat up in use, accounting for some of the thermodynamic inefficiencies of generators and electric motors. Heat can degrade the quality of the magnetic fields of permanent magnets, and so, to stabilize the magnetic strength of iron, small amounts of dysprosium is added to them to stabilize their magnetism under high temperature conditions.
One hears of an "energy transition" frequently as if one exists. It doesn't exist; we are more dependent on dangerous fossil fuels than we have ever been. One hears that dysprosium is a critical element for the mythical "energy transition" and certainly the quixotic quest for so called "renewable energy" does raise demand for dysprosium in wind turbines, where it is stranded when the wind isn't blowing, but generators in dangerous coal powered plants, dangerous natural gas powered plants, and dangerous petroleum powered plants also use dysprosium laced magnets, as do the magnets in hair blowers, fans, electric cars, drink mixers, audio speakers, etc., etc., etc., on and on and on. One can, of course, make magnets from say, just iron, but the cost of doing so will be environmental, the energy efficiency of all of these devices will be lower and thus the environmental impact higher.
The five mines studies leach out about 600 kg, less than a ton, of dysprosium every year. The price of dysprosium in recent times has varied between roughly $400/kg and $750/kg in recent years, but one should note that this represents isolated dysprosium, separated from all of the other 15 elements in the lanthanide fraction including yttrium and scandium. In fact most of the economic value of the lanthanides lies with dysprosium, neodymium, and praseodymium.
Dysprosium demand is roughly about 8000 tons per year.
It is thus doubtful that the cost of recovery systems for these elements can be covered by their sale, but this may not be the main reason for accomplishing the recovery. The reason for removing the metals might be something that is often neglected in discussions of the embrace of technology which are generally only addressed in purely materialistic terms, cost. For instance, advocates of the failed and useless solar and wind industries often like to crow that they're "cheap" even though they require redundant systems which default to the use of dangerous fossil fuels, albeit coupled with soothsaying about vast mining enterprises in the future. (Mines depend on fossil fuels.) Thus the real cost, known as the external cost, the cost to the environment and human health is treated as if it doesn't exist, hence 7 million air pollution deaths a year and a planet in flames. Wind and solar are not "cheap" even if the electricity produced by them for short periods cannot be sold at a decent price, and in fact, no electricity can be sold at a decent and sustainable price, insuring that everybody loses.
Thus I would like to suggest that the recovery of elements from dilute sources like acid mine waste be undertaken for a different reason than satisfying bourgeois affectations: Decency. Future generations will be required to recover metals from dilute sources, waste heaps, and things like this mines. When we mutter obscenely about putative nirvanas (that never arrive) "by 2000" and then when 2000 comes, "by 2020" and then when 2020 comes "by 2040" and so on ad infinitum, what we are saying is that future generations should do what we have not bothered to do ourselves. Hey folks, rather than foist responsibility on our grandchildren, how about we take action ourselves. The side product of removing metals from streams of water is clean water.
This is the same as is the case with uranium. It is cheaper to mine uranium than to recover it from seawater, or from water supplies either naturally or as the result of anthropogenic isolation and processing. However if we remove uranium from natural resources, then we need to mine less or perhaps not mine any at all.
I do not expect an ethical world; at the end of my life, experience teaches me that expecting ethics to dominate decision making is a fool's errand, particularly in the age of the triumph of blank indifferent materialism. This said, an ethical world is feasible if not likely, and thus I like the focus of this paper.
Have a nice evening.
Video graphic, growth and leading nations in nuclear energy 1966-2022
China is now #2. One should note the length of time it took China to go from number zero to number 2, or for that matter, the amount of time it took for the US to go to #1.
OK. I finally did it. I watched 15 minutes of "Jersey Shore."
My son (genetically approximately 1/4 of his genes are of Italian origin) has a new girlfriend, a fellow nuclear engineering graduate student. On learning that he's from New Jersey, where she's never been, she asked if it's really like "Jersey Shore."
We never saw the show, so I rented it, and watched 15 minutes of the first episode with my wife (who grew up on Staten Island).
We laughed a bit, then turned it off.
It was apparently about being proud of being a "Guido," or a "Guidette," body building, considering one's self as incredibly sexy, and drinking. The show is about being young, clueless Americans of Italian extraction who happen to congregate in New Jersey.
Of course, when I was growing up on Long Island, I thought of New Jersey as some kind of hellhole, an opinion I continued to hold when I lived in California, until I moved here.
We were embarrassed because we actually brought our kids to Seaside Heights for family vacations. Trust me, it wasn't like that.
I suppose somewhere in this state there is this kind of reality, but no, that's not us.
New Jersey is a kind of heaven. It takes a while to realize this, but it's true.
The Case for Risk Based Environmental Analysis of Floating Solar Cells.
The paper to which I'll refer in this post is this one: Making a Case for Environmental Risk-Based Monitoring of Floating Solar Systems
Mainak Bhattacharya, Arun Kumar, Arvind Kumar Nema, Sovik Das, and Arya Vijayanandan Environmental Science & Technology 2024 58 (6), 2595-2597
I make no secret of my abhorrence of so called "renewable energy" because of its unacceptable energy to mass ratio, its unreliability, its dependence on fossil fuels, and the bizarre definition by many people calling for the industrialization of wilderness for short lived consumer junk as "green."
I often note that while people have recently begun, despite the data showing its uselessness, to claim that affection or affectation for so called "renewable energy" is about climate change. It is no such thing. The mindless embrace of "renewable energy" has done nothing to address climate change. As I often note, the more money and resources that are squandered on renewable energy - in this waste is growing, not falling - the faster the degradation of the atmosphere is proceeding.
Numbers don't lie: At the Mauna Loa CO2 Observatory, a Terrifying, Startling Week and Month, New Records Everywhere.
One still sees dubious affection for so called "renewable energy" in the scientific literature - which is after all a human enterprise, in no way oracular although guided toward truth by process, but still subject to human flaws - but I have noted increasing questioning of the enterprise in recent years, based on the realities that this reactionary program are now presenting.
Having destroyed and decimated much land mass, now there is active discussion of destroying bodies of water for this affectation, so I welcome this paper's immediate questioning of the enterprise, looking before we leap. We already have wind turbines at sea, the plastic coating on the vanes being known to peel into microplastics, and now we want to add plastic floats for wind turbines.
The authors of this paper note the risks of this ongoing nonsense:
The FPVC typically consist of (1) floating pontoons made of materials like polyethylene (PE), low-density polyethylene (LDPE), high-density polyethylene (HDPE), and pyrocarbon; (2) floating electrical sensors and cables and other electronic equipment covered with PE-type materials that contain trace quantities of heavy metals (Al, As, Cu, Mn, Ni, and Zn) and boron; (3) partially or fully submerged anchors and moors made of HDPE and concrete; and (4) tilted solar panels that are not in direct contact with water but occasionally touch the host water body during high tides, heavy winds, rainfall, washing, accidental breakage of solar panels etc. The composition of the solar panels varies depending on their types. The first- and second-generation solar panels mostly used in the energy sector contain Si, Al, Ag, Pb, Cu, Zn, Cd, In, Ga, Se, Mo, and Te. With respect to all of the aforementioned information, three major environmental concerns might arise. (1) Penetration of sunlight into the host water bodies will be hampered, which will subsequently decrease the chlorophyll content and algal population that might further affect the overall ecosystem of the water body. (2) The dissolved oxygen (DO) concentration below the floating solar panel might decrease, which can affect the aquatic biota. (3) Leaching of microplastics, organic carbon, heavy metals, and metalloids from different components of FPVC might cause contamination in the host surface water body (Figure 1). All of these aspects, along with the steps required for systematic management of FPVC projects, are discussed further here...
The key to getting a question answered is to ask it. I applaud these authors for doing so.
...Because it's not entirely outside the realm of possibility you wanted to know this:
Correction to “Per- and Polyfluoroalkyl Substances in Personal Hygiene Products: The Implications for Human Exposure and Emission to the Environment” Yan Zhou, Xia Lin, Yudong Xing, Xin Zhang, Hian Kee Lee, and Zhenzhen Huang, Environmental Science & Technology 2024 58 (5), 2584-2585
Quantitation of Heavy Metal Release in Urban/Wilderness Interface Fires.
The paper to which I'll refer in this post is this one: Quantification of Bioaccessible and Environmentally Relevant Trace Metals in Structure Ash from a Wildland–Urban Interface Fire Carmen M. Villarruel, Linda A. Figueroa, and James F. Ranville Environmental Science & Technology 2024 58 (5), 2502-2513.
Unfortunately I won't have much time to comment on the paper, but the excerpts I'll provide are telling about the world we're living in because of climate change about which we are doing nothing, nothing at all, except jawboning with absurd and delusional soothsaying driven by wishful thinking.
The results are given in the abstract, more or less. I'll make a few comments on the analytical methods and a little relevant other chemistry after the excerpts.
The excerpts:
Wildfires have a direct impact on air quality. Wildfire smoke contains volatile organic molecules, fine particulate matter (PM2.5, PM10), ozone, aldehydes, sulfur dioxides, and other contaminants, (21?24) which have been linked to increases in overall mortality and respiratory morbidity. (25?32) Hospital admissions increase during wildfire activity (31,33,34) with respiratory admissions increasing 23–34%. (28,35,36) Repeated annual exposure carries an additional risk of long-term illness including elevated risk for developing lung cancer or brain tumors. (37) Wildfires also impact environmental health by destroying vegetation, (38,39) altering animal behavior, (40) and generating ash and atmospheric particulates. Following severe burns, slopes lose the vegetation that prevents erosion, (41) increasing vulnerability to debris flow landslides during rainstorms. (42) Erosion and wind events deposit ash onto soils (43?45) and surface waters, (46?51) thus contaminating water sources (52,53) and increasing sediment load. (54)
Wildfire ash and burned soils are often enriched in trace metals. (43,55,56) Metals can become volatilized at high temperatures during combustion and then condense, subsequently adsorbing to ash surfaces during cooling. (44,45) Studies have also shown that the conditions present during combustion can induce transformations in metal speciation across matrices, including soils, coal, and biomass. (57?61) These alterations to metal speciation can increase the mobility and toxicity of metals, (57,58,60) underpinning the urgency for quantification in environmental systems...
...The Marshall Fire, a WUI fire in Boulder County, was the most destructive fire in Colorado history. Over the course of 2 days, the Marshall Fire destroyed 1084 structures, primarily residential dwellings, and burned over 24 km2. In contrast to wildfires, which burn mostly vegetation, structures are highly concentrated sources of bulk metals, which are present in structural components such as support beams (Ni and Cr in steel), plumbing (Cu and Pb), wiring (Cu), electronics (Cu, Ni, Pb, and Cr), and paint (Cr, Cu, Pb). Nevertheless, exactly how the presence of bulk metals in materials subjected to WUI fires impacts the final metal concentration in ash is poorly understood. To date, there has been little research to determine the composition of ash generated from burned structures and its potential for environmentally and biologically relevant metal release.
Rapid expansion of WUI (18,71) and increase in wildfire activity (12,18,72) will lead to increased quantities of structure ash, and it is imperative to understand the impacts that this may have on human and environmental health. Metal mobilization is of environmental concern due to the persistence, mobility, bioavailability, and bioaccumulative properties of trace metals (43,63) which can be examined through the use of laboratory-based extraction procedures...
The extractions were done in pure deionized water and, in a form of accelerated testing, by acid. The text above refers to speciation, but it appears that speciation was not followed in these procedures, which requires liquid chromatography. LC-ICP instruments are relatively rare, but can be configured; I have some experience with these but in biological settings as opposed to environmental settings. In these systems, speciation, but not oxidation state (other than that connected with the complexation) is not generally available.. The detector here, however, was not ICP (inductively coupled plasma) but rather OES (optical emission spectrometry.) Neither of these techniques can directly determine oxidation state for the metals found, which include copper, an element in the class of having concentration related toxicity - small amounts are essential, but higher levels can be toxic - but of more concern is chromium and lead. I understand, although I have not used it, that ICP/MS/MS can measure oxidation state; people have tried to convince me to buy one, but we don't need it in our lab.) Oxidation state for both is important, more so in the case of chromium. In its lower oxidation states chromium is only mildly toxic, but in its most oxidized state, Cr+6, it is a powerful carcinogen. Lead is toxic in all its oxidation states, but lead forms mostly insoluble halides, including the chloride and the iodide as physiologically important anions.
Anyway, it's illustrative of some of the very toxic side effects of climate change, although in some settings, climate change is deemed - insanely in my view - as less important than whining endlessly about Fukushima. (How many people died from radiation again?)
Have a nice weekend.
The Vogtle 4 Reactor in Georgia USA Reaches Criticality.
Vogtle 4 reaches first criticality.Subtitle:
Excerpts:
The reactor's power output will now be raised to prepare it for synchronisation to the electric grid, and the start of electricity generation. Operators will take the unit through a gradual power increase until it reaches its full power output. Tests to ensure all systems are operating together and to validate operating procedures will be carried out throughout the start-up process before the unit is declared to be in commercial operation.
Unit 4 is one of two Westinghouse AP1000 units being built at the Vogtle site near Waynesboro in Georgia, which is already home to two operating pressurised water reactors. Unit 3 - the first new nuclear unit to be built in the USA for more than three decades - reached initial criticality in March 2023, and began commercial operation in July...
...In its statement announcing Vogtle 4 had reached first criticality, Georgia Power said: "The new Vogtle units are an essential part of Georgia Power's commitment to delivering clean, safe, reliable and affordable energy to its 2.7 million customers. When operating, each of the new units can produce enough electricity to power an estimated 500,000 homes and businesses."
As well as Vogtle 3, four AP1000 units are in operation in China with four more under construction, and two more planned. The design has also been selected by Poland, Ukraine and Bulgaria for their nuclear energy programmes.
Although I'm thrilled that the reactor will soon enter commercial operation, I object to this article's use of "home" as if it were a unit of energy. It is not, but is often treated as such by the media, but it represents a low level of literacy and should not be used if in an attempt to describe reliable clean systems like nuclear power plants, and/or unreliable unsustainable stuff like wind turbines, solar cells and batteries.
The full article available at the link mentions the completion of the Watts Bar 2 reactor after construction stopped for many years. This makes me wonder if it's possible that Seabrook 2 in New Hampshire could be finished and work to save human lives. The partially completed Watts Bar reactor was stopped for over two decades before construction resumed in 2007.
Watts Bar has been saving human lives since 2013.
I came of age in the Early 1970's and became a grown up in the late 1980's with the help of my wife...
...who is actually a decade younger than I am. I was a "late onset" father. My wife were married for almost a decade before we agreed with each other to become parents.
Your "millennial" is slightly older than my first son; your two "Gen Z's" are slightly younger than my youngest son, an MS level materials scientist working on his Ph.D. in nuclear engineering.
My oldest son majored in art, and is working in art, his career is beginning finally to gel; but he lives at home with us; we don't charge him rent so he can sock money away, which he is in fact doing.
Anyway, about me, for some autobiography, I therefore am a (gasp) boomer, and my wife is on the border of being a "boomer" and whatever comes after "boomer," "X," I think.
The worst generation in my view is the one to which I belong, but I would include the "X's" - I believe these distinctions "booms," Xs, Millennials..., will vanish in history - with some responsibility for the wrecking of the planet. We will all wear the stain on wrecking the planet by obliviousness.
We will all be remembered in my opinion to have partied and consumed our way into a planetary disaster and history will not forgive us.
I first caught a glimpse of this somewhere about 1978, when I flew in to my old home from California, and my friends bought tickets to see "Jefferson Starship" in my honor as their guest. It was a few days after Solzhenitsyn gave his speech at Harvard condemning Western "Civilization" for it shallowness, as it were.
Look, it's not like I agreed with Solzhenitsyn that a "spiritual" component is necessary for morality and decency. I'd even argue that his own "morals" and "decency" were distinctly questionable. I'm distinctly aspiritual myself, and whether my "ethics" and level of "decency" are themselves suspect is not for me to say. I'm no philosopher. Still, seeing a bored Grace Slick sing "White Rabbit" for the zillionth time, with a huge American flag hanging from the roof of the Coliseum, with fist fights outside in the hallway and stoners being chased by undercover cops, drunks laying in their own vomit in the parking lot, I was struck with a kind of dystopian horror, a feeling that something was wrong with us, specifically my generation.
I remember that well.
I wasn't even aware of climate change then, and my environmental views were, at that time, nonsensical rote tripe albeit filled with an inflated sense of self righteousness that only weakly masked stupidity and ignorance, the same as what one still sees around, all too commonly.
I decided to change, and awkwardly I moved to do so, in fits and starts. Then by sheer accident, I met my wife, through ogling her with a bunch of other men. Despite all of that, she let me in her life, and she shrugged off my hormonal fascination to tell me who she was, and who she was was something beyond the inspiration of all those male hormones that followed her around, mine and others, moths, flames, all that stuff. She was a human being, a profound human being.
It was pure luck that she stood by me.
Now I'm looking at the end of my life, and my heart is breaking because I could have been, should have been, more but in the end what I did manage, under the general rubric of "too little, too late," sort of makes up for my boomerism, of which, I confess, in the end, I am not entirely cured.
My son in nuclear engineering has led a charmed life, I think, embraced by people with the power to offer him huge opportunities. He works hard, but is quickly rewarded for his efforts. I think he doesn't know of hard work that is not rewarded. The opportunities just keep coming to him and I do my best to remind him of his responsibilities to act nobly with what he's been given.
(I also argue technical points with him, so maybe they'll emerge back in his mind after I am dead.)
I know that many, maybe most, young people are struggling, and the weight on them, financial and otherwise may stem from the fact that their parents abrogated responsibility for leaving a better world than the one they themselves found. Struggle though can be good for one's "soul" whatever a "soul" might be. (My biggest worry for my youngest son is that he has not struggled all that much; a failure to struggle can lead to "asshole disease." He doesn't have it, but he could develop it.)
I nevertheless expect a great generation is rising, and they will find a way to be worthy of their being, even if "we" - the generations of oblivious consumers - were not. Let us not regret their lives, but instead limit any regrets to our own lives, such as they were.
Thanks for asking.
From Retraction Watch: "I know imitation is the sincerest form of flattery...
Retraction Watcnh is a wonderful website that monitors retractions in the primary scientific literature.
I was reading this article: 'The sincerest form of flattery’: How a math professor discovered his work had been plagiarized... in which a Math Professor learned that one of his works had been plagiarized.
I found a quote from the professor amusing:
I bolded the part that amused me.
It's in the psyche of the world that Trump is a pathetic liar.
I must really be looking old and wrecked tonight.
I'm at the airport and checked in early, stopped for a vastly overpriced beer after getting through security, mostly because I needed to sit to rest my bad back and hip.
I went to the gate on my boarding pass only to find the flight there was to Chicago whereas I'm going to Boston. The gate changed from concourse B to concourse A.
It was quite a walk, and the pain started rising, but I would have made it with a few sitting stops. Then one of those shuttle cart drivers took a look at me and invited me to get on. She drove me to the gate. When I got there, all the seats were full, but a nice young man offered me his.
On one level the kindness is thrilling; on another I wonder how terrible I look.
The days when I could sprint across airports for connections are over.
I am happy to have lived a long time and if the price is a little manageable pain mixed with human kindness, well I'm a lucky guy.
Happily I don't have to travel as much as in younger days.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,586