NNadir
NNadir's JournalHighly Selective Separations of Actinide (and Lanthanide) Elements Using HOPO Ligands.
The paper I'll discuss in this post is this one: Ultra-selective ligand-driven separation of strategic actinides (Rebecca Aberget et al Nature Communications 10, Article number: 2438 (2019)
(If you are interested in this paper, but less interest in my commentary sarcasm and spin, the paper is open sourced and you can read it yourself.)
Some history: In 1944, a fellow named Don Mastick, a 22 year old fellow working on plutonium isolation from the Manhattan project, accidentally swallowed much of the world's supply of isolated plutonium. His story is covered extensively in Eileen Wellsome's book The Plutonium Files: America's Secret Medical Experiments in the Cold War, which is available in many public libraries and places where you can buy books. It's worth reading. Masticks job after swallowing plutonium involved to recover precious plutonium from own his feces and urine, a job he didn't like, and later was able to transfer to a job as a technical accompanist to the bomb parts when they were brought to Tinian to be dropped on Japan. Don Mastick died in 2007; he was 87 years old and for much of his long life was interviewed from time to time about his plutonium eating adventure. Mastick represents the first known case of human ingestion of plutonium.
From the late 1940's through the early 1960's ton scale quantities of plutonium were deliberately vaporized in the planetary atmosphere, probably forming fine aerosol droplets after condensing as a liquid on bomb fragments. Plutonium, among all elements has the third widest temperature range in which it remains liquid, exceeded only to neptunium and gallium. As free unionized oxygen migrated into the blast plasma into the cooling blast core, this plutonium oxidized, probably to PuO2 where it fell, as dust, as a component of "nuclear fallout." If you were born after 1946, you have been living with components of this fall out your entire life, with said concentrations probably peaking around 1963. Concentrations of radioactive elements released by nuclear bomb testing can be pretty much be detected everywhere on Earth, and are often used as tracers for things like water flows, the age of sediments, rates of soil erosion, melt rates of glaciers, etc.
As a result of industrial and war like exposure to actinides like plutonium, and in an effort to make radiotherapeutic drugs for cancer patients - still very much an active area of research - scientists became interested in physiological chelation of plutonium in biological systems, particularly with a goal of removing from animals and humans, in cases of accidental or experimental (or war time) exposure.
As I was researching the origins of the HOPO ligand mentioned in the title of this post, I came across a review article in my files where it is discussed in some detail, this one:
Rational Design of Sequestering Agents for Plutonium and Other Actinides. (Patricia Durbin et al Chem. Rev. 2003 103 11 4207-4282) Here's a graphic from that paper in which the scheme for the synthesis of HOPO is presented, using spermine as starting material:

Spermine, named because it was first isolated from human semen, is a constituent of all eucaryotic cells, where, among other things, it serves to stabilize the geometry of DNA. Metabolically it derives from the decarboxyation of ornithine, itself derived from the genetically coded amino acid arginine by deguanidation.
Anyway. Patricia Durbin, one of the authors of this review, passed away in 2009 at the age of 81. She was an important scientist working at UC Berkeley/Lawrence National Laboratory on the biological chemistry of the actinides, and was considered one of the most prominent health physicists of her time.
Her scientific obituary is here: Dr. Patricia Durbin
Here is an early paper in which she published about the discovery of the HOPO ligand:
Specific Sequestering Agents for the Actinides. 28. Synthesis and Initial Evaluation of Multidentate 4-Carbamoyl-3-hydroxy-1-methyl-2(1H)-pyridinone Ligands for in Vivo Plutonium(IV) Chelation (Durbin et al J. Med. Chem.1995 38 14 2606-2614)
Publication in the Journal of Medicinal Chemistry shows that this ligand was originally designed for medical treatment of people who, like Don Mastick, are unintentionally (or deliberately) exposed to plutonium in order to remove it. It appears however, that the utility of this compound extends to possible industrial use, hence the paper cited at the outset of this post, the Nature Communications paper.
From the introduction to the paper:
After discussing the difficulties of the current approach to actinide separations, which are currently only industrial in liquid/liquid extraction settings, and detailing the difficulties of designing extractants for these purposes, the authors continue:
Actinium-225 is considered to be a very important tool in cancer therapies, but it's availability, which involves bombardment of thorium with high energy protons at Oak Ridge National Laboratory, has been limited, in part by the difficulty in separating it from a wealth of side products in what is essentially a proton driven fission event.
Actinium-225 has a half-life of about 10 days, and rapidly decays, through a number of daughter atoms to bismuth-209, the metastable natural isotope of this monoisotopic element. Attached to a ligand designed to bind to cancer cells, it effectively kills them.
Here's a graphic from the paper demonstrating some of the chemistry of this remarkable ligand:
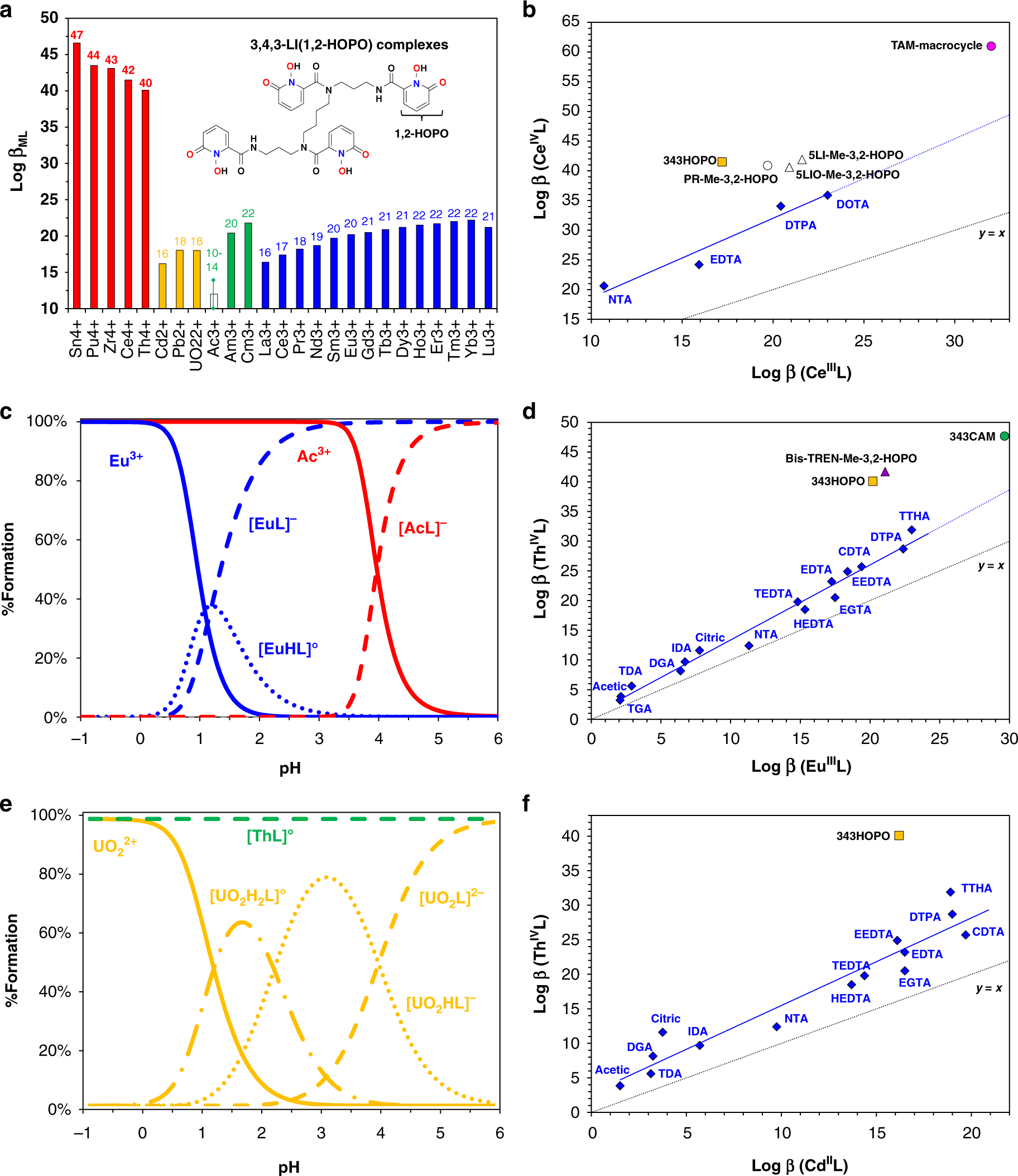
The caption:
I will not produce more texts or graphics from this most interesting paper, since, again, if interested, one can easily access the full paper since it is open sourced at the link above.
Let me say this: In my long considered opinion, the only viable path to addressing climate change passes through the chemistry of the actinides, and indeed the only viable path to getting access to nuclear weapons out of the hands of crazy people by eliminating all or most access to everyone, sane or otherwise - particularly as we can see that a psychopath with a weak education, poor moral development, with puerile emotions and marginal intelligence has gained access and control over the US Nuclear Arsenal - is to make more plutonium, not less of it.
I advanced an argument of why this is true elsewhere: On Plutonium, Nuclear War, and Nuclear Peace, and, although I have expanded my views on what I wrote six years ago, this covers the basic reasoning. It is absolutely critical, pun intended, that we increase world inventories of plutonium-238 and plutonium-240 to minimize the potential for nuclear war.
In doing this, we can also eliminate, using the uranium already mined for centuries, the use of dangerous petroleum, dangerous coal and dangerous natural gas.
The chemistry of the HOPO ligand, after the fashion of medicinal chemistry's SAR approach can be modified in a number of ways to make things like liquid membranes to further refine these separations. To me this interesting expansion on the utility of this ligand from its original purpose of in vivo decontamination to potential industrial utility is worthy of deep consideration.
This work explores the possibility of using simple and clean redox chemistry, including but not limited to, electrochemistry, to provide what may be a continuous process (as opposed to batch processes) for the recovery of valuable elements from used nuclear fuels.
This is important and creative work.
I trust you're having a pleasant weekend.
In a brief vacation I saw two very moving historical sites.
My family took a quick run down to Oak Ridge National Laboratory to drop my youngest son off for his summer internship, and as this laboratory, constructed in a massive effort as part of the Manhattan project, is not only a great laboratory but an important point in American history and the history of science, we decided to take the public tour.
I almost certainly knew more of the technical stuff than the guides did, but still, it was a remarkable thing to see and hear about, Lawrence's Calutrons, which apparently were more effective in the early 1940's at separating isotopes than the famous K-25 plant, which has now been demolished, although the tour gives you a feel for the size of the thing.
Most incredible was the part of the tour which involved seeing the core the world's first continuously operated nuclear reactor, now the "Graphite Reactor National Historic Site" which is the actual core, replete with some mannequins who are eternally pushing fuel through the core with mechanical rods. You could go to the control room, a primitive affair with chart recorders. This is the actual reactor that operated for 20 years to make plutonium for warheads, including plutonium for the Nagasaki and Trinity bombs.
Then on the way home, I convinced my wife and son to stop off at the Antietam battlefield, which the ranger reported as the best preserved Civil War battlefield. The ranger in question gave a lecture on the battle with tremendous passion and although I have read a great deal about the battle, it is something quite different to hear it described while looking at the field from the visitor center, which is located in the exact place where Robert E. Lee, a combatant commander for the slaveholders, stood during the battle as he sought to establish slavery as a permanent and irrevocable feature on North American soil. The Ranger noted that the tactical draw that the battle represented was claimed by Abraham Lincoln as a victory, since Lee was forced to retreat to Virginia, giving Lincoln an "excuse" to issue the Emancipation Proclamation that changed the war from an effort to preserve the Union into an effort to make the Union worthy of its ideals of putative freedom.
When I walked that field, I wept a little, for the men who died there, for the more than 100,000 who lived through the horror of that day. The Ranger told us that they are still finding the dead on the field, the most recent soldier discovered in 2008, a New York soldier (identified as such from the remains of his uniform) who was, at the request of the State of New York, repatriated to New York, after 147 years, and buried with honor in the Saratoga National Cemetery.
Modern forensics showed he was about 19 when he died, a year younger than the son I dropped off at Oak Ridge.
The Antietam battlefield to this day marks the site where the most Americans were killed, wounded or missing in a single day, more than D-Day, more than Tarawa, Iowa Jima, the Battle of the Bulge. From the visitor center you can more or less see everything, the whole panorama of a tragedy, a necessary tragedy, but a tragedy all the same.
Five intense days, setting my son up, touring the historic features of the great National Laboratory where great science is still being done (and in which my son is participating) and seeing the exact place (more than Gettysburg) where the "New Birth of Freedom" actually was released in rivers of blood.
It was all the more horrible to recognize that a horrid ignorant racist, a member of what is only nominally Lincoln's party is in the White House, spitting and drooling on the history of our country.
Joint Formation of 1,2 Propylene Glycol and Isopropyl Alcohol from Biologically Derived Soketal.
The paper I'll discuss in this post is this one: The Joint Synthesis of 1,2-Propylene Glycol and Isopropyl Alcohol by the Copper-Catalyzed Hydrogenolysis of Solketal (Vadim O. Samoilov,* Denis S. Ni, Georgiy S. Dmitriev, Leonid N. Zanaveskin, and Anton L. Maximov ACS Sustainable Chem. Eng. 2019, 7, 9330?9341)
I'm not all that much into - in fact I generally oppose - so called "renewable energy" because of its land use and material intensity is in my view, environmentally unacceptable. The failure of this strategy is recorded in the planetary atmosphere, CO2 concentration increases are accelerating, not falling, and in the fact that the use of dangerous fossil fuels and the resultant death toll are also rising not falling. Reliance on this strategy, while remaining popular in many places, is making things worse, not better.
It was not always so with me; I once thought - not all that long ago - that so called "renewable energy" was a good idea. In particular, I was very fond of the idea of biodiesel at one time, until, at least, little trivialities like the destruction of Indonesian rain forests to make palm oil plantations.
Driven by largely German "Renewable Energy Portfolio Standards" biodiesel was, for a time, a very popular fuel, although, according to the authors of this paper it no longer is, since they remark in their Russified English the following:
Glycerol is a by product of biodiesel production, and although it has always had some commercial value, prices for it fell so dramatically in the "biodiesel boom" that it ended up being dumped, which is a shame, as it has a significant amount of concentrated carbon removed from the atmosphere.
I personally believe that biomass offers something of a path to removing carbon dioxide from the atmosphere, something that will fall to future generations as we have screwed them - with our dangerous fantasies - out of their rightful inheritance of a clean planet, albeit one that will always remain minor, but I favor a brute force approach of high temperature reforming driven by nuclear heat rather than expensive and water intensive fermentation processes. This said, there are some applications where biological schemes might be utilized to obtain specialty chemicals without too much strain on the environment, and, as I have been interested in biodiesel in the past, and in glycerol, this paper caught my eye.
"Soketal" is a compound made by condensing acetone with glycerol. It's structure will be shown below. About half a century ago, industrial operations of the "ABE process," a fermentation process, (Acetone, Butanol, and Ethanol) operated in some countries commercially. Today most of the world's acetone is made from the oxidative decomposition of cumene, a product of the dangerous petroleum industry, butanol from the oxidation of butene from the dangerous fossil fuel industry, and ethanol from corn, albeit, in the United States, at the cost of the complete destruction of the Mississippi River Delta's ecosystem.
The paper before this one in this journal is about reviving the "ABE Process." (It's probably a bad idea.)
Anyway, from the paper, the full introductory paragraphs excerpted above:
Regarding the methods of production of propylene glycols (PG), scientific efforts are mainly aimed at (a) the development of new heterogeneous catalysts for glycerol conversion providing enhanced stability and selectivity toward 1,2-PG and (b) the development of approaches toward the selective hydrogenolysis of glycerol into valuable 1,3-propanediol.(1) While the noble metal-based catalysts have turned out to be the most promising for the latter purpose,(2,3) the copper-based catalysts seem to be the best choice for the hydrogenolysis of glycerol into 1,2-PG because of the relative low costs and efficient performance. High yields (up to 98%) and selectivities (up to 99.6%) in glycerol hydrogenolysis have been reported for Cu,(4?10) Cu–B,(11,12) Cu–Cr,(5,6,10,13) Cu–Mg,(14) Cu–Pd,(15) and Cu–Zn(16,17) catalysts.
At the same time, much attention has been recently paid to glycerol acetals and ketals, due to the relative ease of synthesis and intriguing prospects of application as fuel additives(18?20) and solvents.(21) The majority of the investigations are dedicated to solketal (2,2-dimethyl-4-(hydroxymethyl)-dioxolane-1,3), a ketal formed by the condensation of glycerol with acetone. For the synthesis of this compound, a few technological schemes have been proposed,(22?24) but one of the most interesting points about solketal is that it could be synthesized directly from crude bioglycerol.(25?27) A specific feature of the process (using sulfuric acid, sulfonic exchange-resins, or AlF3·3H2O as a catalyst) is the relative ease of the crude bioglycerol conversion and the product recovery (the boiling point of solketal is about 187 °C, which is approximately 100 °C lower than for glycerol). Upon the acidification of crude glycerol with H2SO4, a separate lipophilic phase (which mainly consists of fatty acids) is formed. During the ketalization, the major part (?80%) of the mineral impurities is separated as a solid, as well; in this manner, along with ketalization, some purification is conducted.
The authors investigate catalysts for the conversion of soketal into value added chemicals of possible industrial importance.
Some pictures:

The caption:
Almost of the chemicals shown in the following scheme are of some industrial importance, at least if purified, something which is energy intensive, but still...

The caption:
Some yield and distribution information in differing conditions:

The caption:
More yield information:

The caption:
Nothing here is going to be earth shattering, unless an attempt is made to overuse it, but I can certainly see niche applications for this sort of thing, and to the extent carbon is removed from the atmosphere, it's a good thing.
Toward High Level Modeling of Fluids: Application of Laws of Corresponding States to SAFT Equations.
The paper I'll discuss in this post is this one: Application of the Corresponding-State Law to the Parametrization of Statistical Associating Fluid Theory (SAFT)-Type Models: Generation and Use of “Generalized Charts” (Romain Privat,*,† Edouard Moine,† Baptiste Sirjean,† Rafiqul Gani,‡ and Jean-Noël Jaubert, Ind. Eng. Chem. Res. 2019, 58, 9127?9139)
After Chernobyl blew up in 1996 - Chernobyl was and is an example of the worst case in the use of nuclear power, although there is, these days, a lot of popular bad thinking about Fukushima and focus on it - I changed my mind about nuclear energy, and began to consider, in light of the fact that the worst case was now a known, and not a speculative unknown, that nuclear energy is the only acceptable form of energy there is, given the magnitude of the environmental crisis we are experiencing, whether we really care or not.
For people traveling the route I traveled, evolving from dumb-assed, ignorant, anti-nuke to nuclear energy advocate, in my case, a fierce advocate, it seems to me that there is a phase in which one becomes enamored of liquid fluoride molten salt reactors, often referred to by the abbreviation LFTR, for liquid fluoride thorium reactor. This fascination sometimes involves the belief that thorium is an acceptable nuclear fuel whereas the plutonium/uranium system of fuels is not, something with which I disagree, although certainly went through this phase myself.
Around the time I was going through my LFTR phase, I decided to raise my level of understanding of nuclear technology to a higher level, applying my general educational background to expanding my knowledge, and consider in detail the chemistry of nuclear fuels, in the process, learning all about some very interesting things and discovering significant gaps in my knowledge.
I am not really a molten salt kind of guy these days, although I wish all the molten salt people well, but am now more of a "breed and burn" kind of guy, exploiting a very different type of molten fuel. I am still interested in molten salts, not really in power production settings, but as a useful tool for recovering valuable resources from used nuclear fuel, often described by people - excuse the oxymoronic statement - who simultaneously lack imagination and have overactive imaginations, as "nuclear waste."
The chemistry of used nuclear fuels, and nuclear fuels undergoing use, is decidedly complex and represents a scientific challenge inasmuch as nuclear fuel is a continuously evolving substance and by definition, will contain a broad array of elements, some elements from the fourth period of the periodic table, all of the elements in the fifth period, some of the elements in the sixth period, all of the light lanthanides, as well as many, depending on design, of the light actinide elements, in addition to additives, which, again depending on design, might include lithium, beryllium, fluorine, structural elements such as iron, cobalt and nickel and moderators like carbon and hydrogen.
Recently I was looking into the failure of a molten salt nuclear startup which was funded, in part, by a gay Trumper - speaking of oxymorons - run by two MIT graduates, a startup which seems to have advertised a notion that the founders cheerfully admitted to be mistaken after further analysis before disbanding the company and releasing its IP. I decided to leaf through the graduate thesis of one of the founders, and her thesis involved modeling of molten salts got me thinking about equations of state for liquids, which brings me to a discussion of the paper referenced at the opening of this post.
From the introduction to the paper:
As a well-known result, the classical van der Waals, Peng–Robinson (PR), and Soave–Redlich–Kwong (SRK) models obey the corresponding-state law. For a pure substance, these equations of state (EoS) can be written as a universal relationship between the state variables Tr (the reduced temperature), Pr (the reduced pressure), and vr (the reduced molar volume):



where Tc is the critical temperature, Pc the critical pressure, and vc the critical molar volume. For the SRK and PR EoS, an additional dimensionless size parameter (the acentric factor ? ) is added in the universal relationship, which can be written in the general form Pr = f(Tr,vr,? ). It is said that the SRK and PR EoS obey the three-parameter corresponding state law.
To apply such models to a given pure species, one must switch from a space of dimensionless reduced state-variables (Tr,Pr,vr) to a space involving dimensional state variables (T,P,v ). To do so, these EoS require knowledge of three input parameters: the critical temperature (Tc,exp), critical pressure (Pc,exp), and acentric factor (?exp ).
What about SAFT EoS? Derived from molecular-potential models, these EoS consider, as input variables, the ones used in the underlying molecular-potential models. For most of them (PC-SAFT, CK-SAFT, SOFT-SAFT, ...), these are segment number (m), segment diameter (? ), and energy parameter (? ), described below. Therefore, most SAFT-type EoS also obey the three-parameter corresponding-state law. When dealing with this class of models, the reduced state variables involved in the corresponding-state law are normalized using combinations of the input model parameters (m, ?, ? ). In this molecular approach, the reduced temperature, pressure, and density are denoted as T*, P*, and ?, respectively. In their universal form, SAFT-type EoS for nonassociating pure species obey the three-parameter corresponding-state law and, therefore, they can be written as a universal relationship between the three aforementioned reduced state variables and the size parameter m. As a consequence, it can be shown that
the reduced critical coordinates of a critical point are only dependent on the m parameter,
the reduced coordinates of a liquid–liquid–vapor triple point are only dependent on m (note that SAFT-type EoS are known to predict triple points, although this behavior is physically meaningless(1?3)), and
the acentric factor of a pure component is only dependent on m.
These observations can be of special interest when thinking about the parametrization of SAFT-type EoS. The present article aims at discussing two potential applications of such results in terms of EoS parametrization.
(SAFT refers to "Statistical Association Fluid Theory" and PC-SAFT refers to "Perturbed Chain Statistical Association Fluid Theory" )
The paper makes reference to the more familiar gas laws in a nice review, focusing on the general correspondence principles with which they are associated. To wit:
(1)

s an example, the well-known van der Waals EoS obeys the corresponding-state law:
(2)

s highlighted by eq 2, two parameters among the three reduced variables Tr, Pr, and vr must be specified to apply the corresponding-state law. Equivalently, it can be said that two input parameters, to be chosen among the critical temperature, pressure, or molar volume, must be preliminarily known to apply eq 2 to a specific compound. For illustration, the pressure expression provided by the van der Waals EoS is
(3)

Consequently, experimental values of the critical temperature Tc,exp and critical pressure Pc,exp must be preliminary known to apply the van der Waals EoS to a given pure compound.
As a limitation, it is generally observed that two size-asymmetric pure fluids having the same Tr and Pr deviate significantly from the two-parameter corresponding-state law. To overcome this issue, an additional parameter must be introduced. The three-parameter corresponding-state law frequently introduces the acentric factor or the critical compressibility factor as an additional parameter. The universal relationship between reduced variables then becomes

As an example, the well-known Soave–Redlich–Kwong (SRK) EoS(10) obeys the three-parameter corresponding-state law:
(5)

with

where the acentric factor ?exp is considered to be an experimental property, since it is a straightforward function of experimental quantities (i.e., the vapor-pressure at Tr = 0.7 and the critical pressure). Consequently, knowing the experimental values of three input parameters (Tc,exp, Pc,exp, ?exp) is a prerequisite to apply the three-parameter corresponding-state law, as illustrated with the SRK EoS.
(6)

The three-parameter corresponding-state law entails that reduced vapor–liquid equilibrium (VLE) properties depend solely on the reduced temperature and the acentric factor:
(7)

where Prsat is the reduced vapor pressure and ?vapH is the enthalpy of vaporization of pure species.
...and so on. (A well known descendent of the SRK equation of state is the Peng-Robinson equation of state, to which this type of thinking applies.)
What the authors seem to be after is building a database for physical chemists like those that exist, for example, for protein chemists, which has the experimental data readily available for use in complex fluid systems, for example, fluids containing the valuable components of nuclear fuels.
Some pictures from the text:

The caption:

The caption:


The caption:

...and so on...
Here's a nice figure showing the power of systematization:

The caption:
The authors note in their conclusion:
Parameterization methods for associating compounds will be proposed in the future.
That is something to love, scientific and engineering ambition.
(There is so much to love in life, despite the tragedy of our times.)
It's a lovely paper for inducing reflection. It's a shame that I won't live long enough to really know it intimately. Well, my sons at least are smarter than I am.
I trust you're enjoying this weekend. For me, it's a little noisy in this library, as it's reunion weekend and people are walking through it, talking loudly about their old days, but the remaining days are too beautiful to look back all that much.
Doublethink in science. Coal is a renewable resource?
In issues in energy and science, there is, especially in the public, a whole lot of what George Orwell called in 1984 "doublethink."
I'm old enough to know all about 1984 and Orwell, but it's possible that this nightmare book is less well known than in modern times than it was in my time.
For the uninitiated:
Wikipedia "Doublethink"
On the right, we have the absurd notion for instance, that the orange fool is advancing patriotism and, for what it's worth, Christianity.
On the left, we have the notion that wind power represents "green energy," "sustainable energy," and "renewable energy," when, as I have shown elsewhere in this space, the coal required to make the steel for the posts holding these monstrosities up in a case where they represented significant energy, would amount the addition of massive amounts of CO2 emissions, on the order of current annual emission rates for everything (roughly 35 billion tons of CO2 per year) for the steel alone, never mind the copper, lanthanides, concrete, etc... And of course, this infrastructure would need to be replaced every 20 years or so.
These are both examples of doublethink, on one hand, an appeal to belief, on the other, an appeal to ignorance of the details.
Anyway, I came across this paper in my general reading and opened it since I am always thinking about ways of getting rid of the industrial chemical requirements associated with dangerous petroleum:
A Preliminary Study on the Role of the Internal and External Surfaces of Nano-ZSM-5 Zeolite in the Alkylation of Benzene with Methanol (Ji Qian, Guang Xiong, Guang Xiong, Jiaxu Liu, Chunyan Liu, and Hongchen Guo, Ind. Eng. Chem. Res. 2019, 58, 21, 9006-9016.)
It contains this precious remark:
The bold and italics are of course, mine.
The authors are Chinese, and one hopes that it's just bad grammar in translation, a misplaced modifier and really not "doublethink."
These days, one never knows.
I trust you're having a pleasant weekend.
If you believe in the Constitutional Requirement to Impeach Criminals in the White House...
...have you sent a message to your congress person stating that Article 1 Section 5 of the Constitution was written for the likes of Donald J. Trump?
I just emailed mine, Representative Bonnie Watson Coleman.
It takes a few minutes to do. If you feel as I do, please do it.
On the Road to an HIV Vaccine: Stimulating B Cells to Recognize HIV-1 V3 Glycans.
The paper I'll discuss in this post is this one: Immunization expands B cells specific to HIV-1 V3 glycan in mice and macaques (Nussenzweig et al Nature, Published On Line May 2019.
Recently I remarked in this space that when I was a stupid kid, I had a poor appreciation of the beauty and complexity of biologic molecules built from simple building blocks, i.e. peptides and proteins from amino acids, polynucleotides from nucleic acids, and, the topic of this paper, sugars.
Sugar chemistry has been difficult - for me at least - because it is kind of hard to represent on paper. For example, there are 16 "aldohexoses" - glucose being the most familiar - all of which have exactly the same formula, C6H12O6, differing only in their arrangements in space, i.e. their stereochemistry. Moreover, as a practical matter, each of these exist as a pair of "anomers" in which the stereochemistry at a single site shifts chemically, usually with two forms existing simultaneously.
Consider the three most common biological sugars:
Mannose:

Galactose:

Glucose:

Then consider that there are four forms of D-galactose:

...and since there are two forms of galactose which are mirror images of each other, eight kinds of galactose overall.
I was relieved of a small portion of my stupidity some years back when I stumbled upon a wonderful monograph, to which I've referred before in this space, The Sugar Code which has a heartwarming encouraging excerpt in the introduction stating that (I paraphrase): "No, your not as stupid as you think you are, sugar chemistry is hard"
The point of The Sugar Code is that this complexity of sugars, especially when they are linked together, particularly utilizing different sugars in one of two possible linkage systems, represents a remarkable opportunity to store information, an ability that living systems exploit extensively.
Modern advances in technology have made possible to interrogate these structures in hopes of understanding the signals they represent; recently I have been discussing this and other related topics in molecular biology with the major manufacturers of high resolution mass specs, and I'm amazed, totally amazed, at how remarkable this technology actually is.
An important tool for signaling in the immune system are called "glycans" which are an array of polymeric sugars and aminosugars that attach themselves to proteins, notably antibodies at N197, at asparagine (N-glycans) on the ? amide nitrogen, and serine (O-Glycans). The former are easier to analyze than the latter, although great strides have been made for the latter.
This brings me to the paper I cited at the beginning of the post. Signaling in the immune system is often highly involved in glycan signalling, and the text makes clear that antibodies evolve from one another in order to fight pathogenic infections with viruses, particularly those which are retroviruses, the mechanism for transcription in these offering many opportunities for error, and thus evolution.
In the language of the paper's introduction:
Sequential immunization to guide the development of bNAbs was demonstrated in genetically modified mice that carry inferred germline precursors of human bNAbs8,9. However, the priming immunogens that were used to initiate the response failed to activate and expand B cells that expressed inferred bNAb precursors in animals with polyclonal antibody repertoires. Thus, a goal of HIV-1 vaccine development has been to design immunogens that recruit B cells that express bNAb precursors into germinalcentre reactions in animals with polyclonal repertoires.
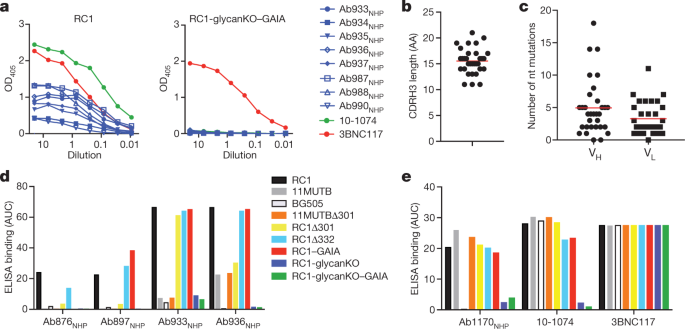
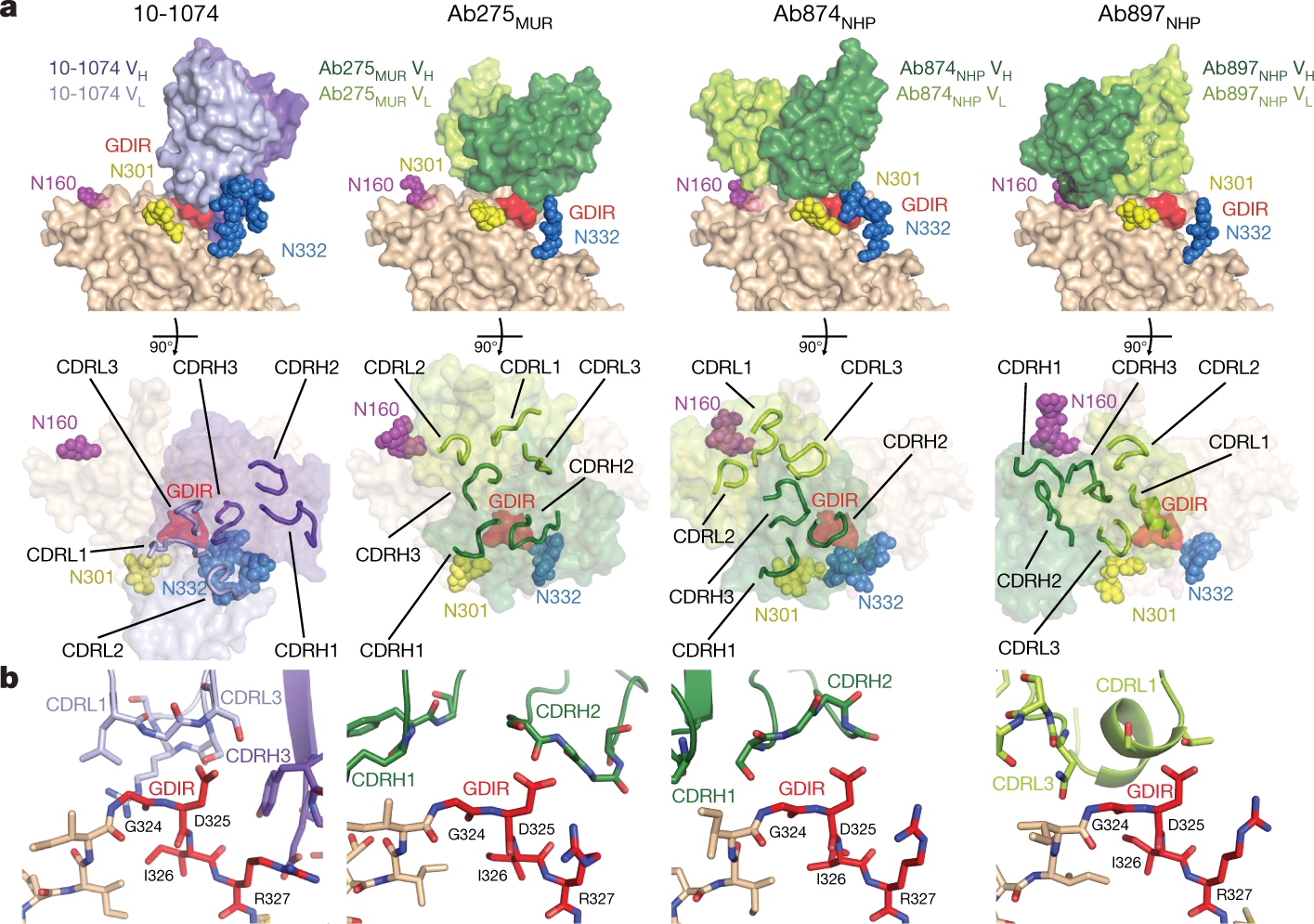
The authors have generated a B-Cell priming immunogen, which they call RC1, and explain in what might seem jargon to the uninitiated:
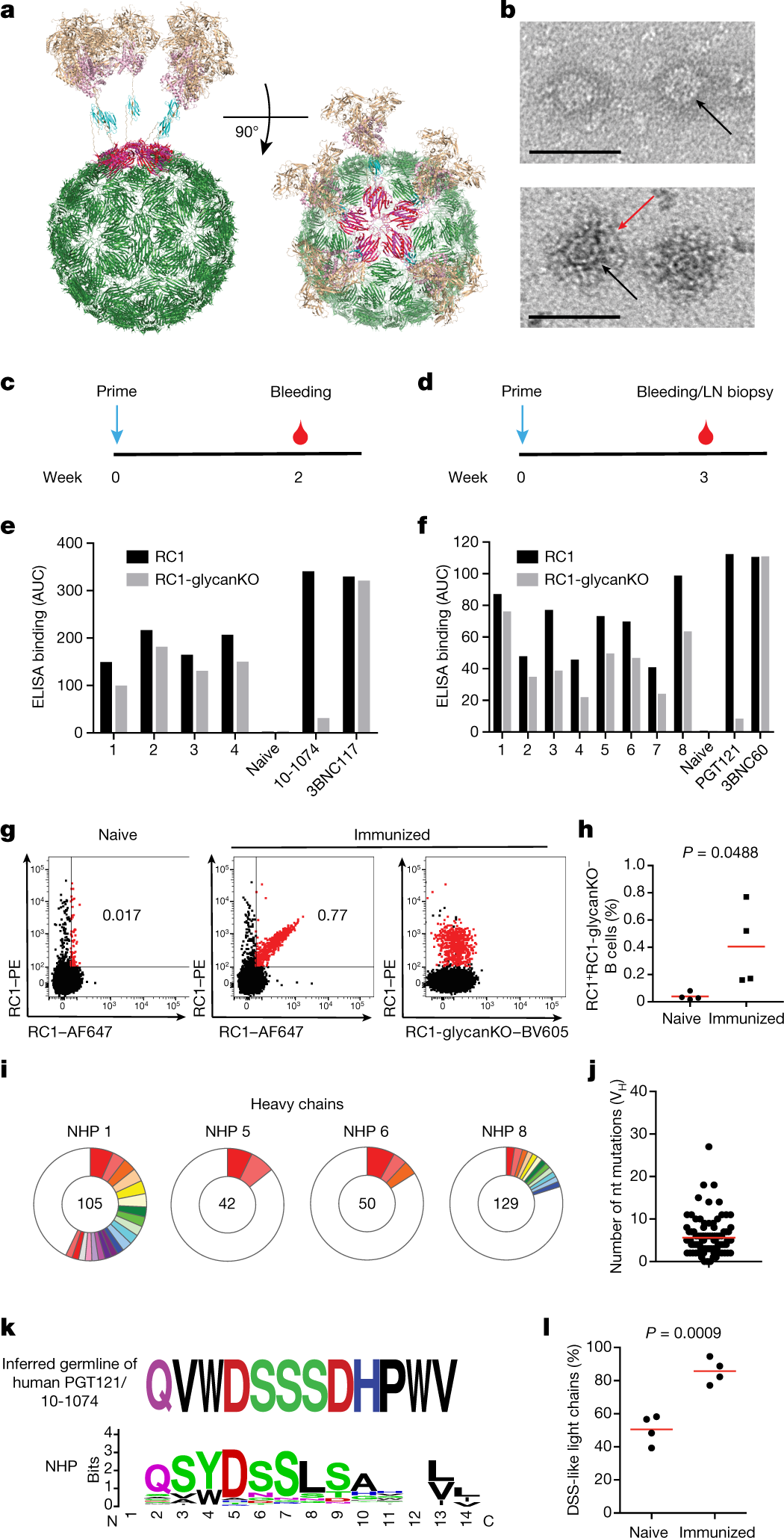
Some pictures from the text:
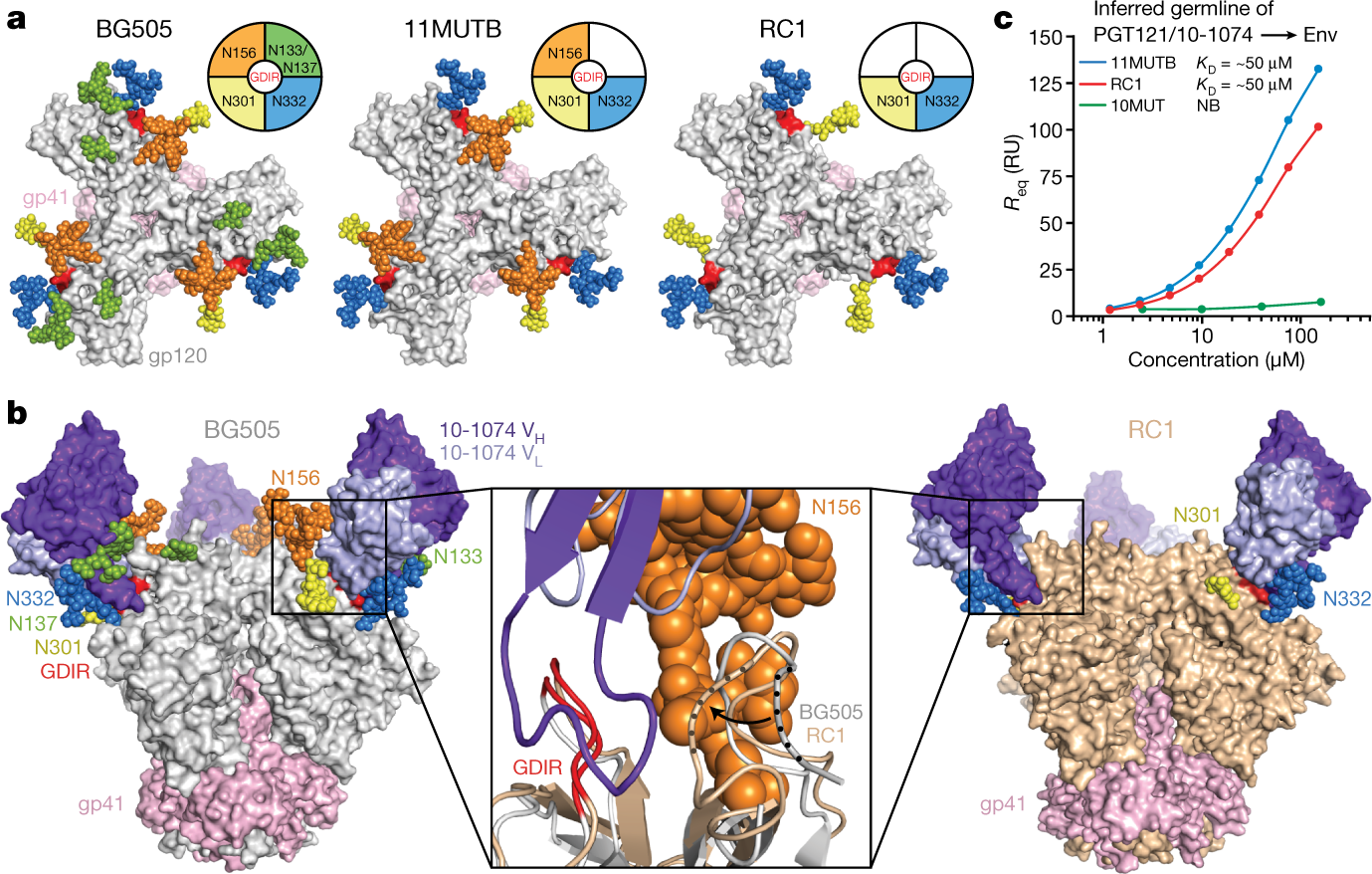 ?as=webp
?as=webp
The caption:
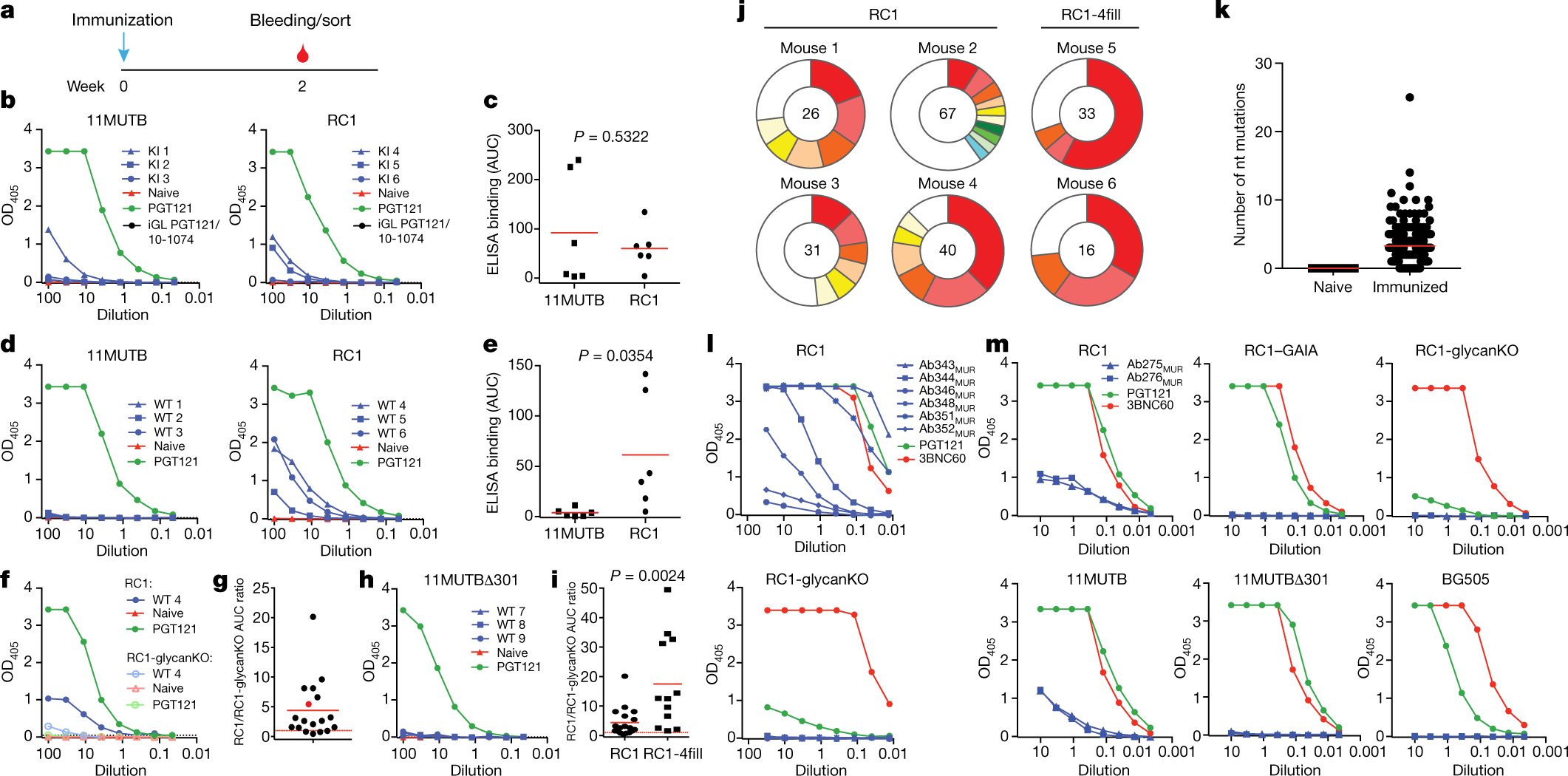 ?as=webp
?as=webp
The caption:
 ?as=webp
?as=webp
The caption:
 ?as=webp
?as=webp
The caption:
 ?as=webp
?as=webp
The caption:
Some comments from the conclusion:
...The principles we used to produce RC1 did not take affinity for a germline B cell receptor into account. Instead, RC1 was designed to increase the number of bNAb progenitors that compete for entry into germinal centres by making the antigenic target site more available and facilitating binding to electrostatically neutral inferred germline precursors25. In addition, VLP-RC1-4fill incorporates the idea that masking competing off-target epitopes26,29 by addition of glycans27 and tethering the bottom of the trimer to a VLP minimizes competition for entry into the germinal centre.
RC1 differs from other HIV-1 vaccine candidates in that it induces B cells that express antibodies against a targeted epitope to undergo clonal expansion in germinal centres in animals with a fully polyclonal B cell repertoire...
...Notably, biochemical and structural results showed that antibodies with distinct mechanisms of targeting the V3-glycan patch were elicited by RC1, increasing the probability that one or more might develop breadth and potency after boosting9. Thus, VLP-RC1-4fill is a suitable candidate immunogen for further evaluation in sequential vaccination strategies to elicit bNAbs.
The development of vaccines has allowed for the total elimination of what was once a widespread fatal disease. The rise of and celebration of ignorance by the ignorant has made this goal less accessible than it was in former times, but with that caveat aside this is some very beautiful molecular biology.
Have a nice weekend.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,580